Nitrates in products: Swiss stores vs Russian stores vs summer cottage
Food safety, as well as food safety, are two cornerstones of a calm and stable life in any country. If in the second paragraph the completeness of information is hidden from prying eyes and is accessible only to people with tolerances, then the first paragraph is a quite measurable quantity. For example, you can measure heavy metals in food and water, you can toxins, or you can just nitrates ( aka NO 3 - ).
Many believe - frankly, not unreasonably - that in Switzerland, food products are the best of the best in the world. Although in 2016 I published a translation of an article in which the problems of this European country with food were quite openly discussed. In 2018, he acquired a “nitrate meter”, succumbing to advertising, from one well-known company and began to measure everything in a row. As a result, in 2019 - in the international year of the periodic table - the idea was born to write this article, where to compare Swiss products from different stores with Russian "analogues", as well as add country products to them and check how environmentally friendly they are.
In this article, we will consider the topic of measuring and the presence of nitrates in products from the point of view of a chemist and even an electrochemist (measurement is still electrochemical).
Background
It’s worth mentioning right away that many copies were broken about the topic of measuring nitrates in products. It is not unreasonably considered that it is practically impossible outside of laboratory conditions and all nitratometers are pure profanation.
For example, in this article already from 2010, the authors compare the devices from SOEKS and Vitatest and come to the disappointing conclusion that nitratometers can distort real indicators several times under certain conditions. We will separately discuss the reasons for this below.
Similar conclusions come from the Aquafor company blog , where the pros and cons of TDS meters were reviewed.

Habra readers also do not particularly favor advertising publications of nitratometers. Both found reviews are found deep in the red-sleeper zone ( one and two ). In the last article, they even tried to measure the nitrate content in baby puree. Although the latter is often stabilized by antioxidants and, in principle, can be considered aka processed food. And you just cannot measure it with a nitrate meter.
There you can also include a review of the Greentest nitrate meter , and some really strange devices .
There were also reviews of the Pribluda for the iPhone, I-la portable and stylish meter of nitrates, radiation, temperature and humidity and everything else in
Well, let's try to figure it out in order.
Introduction: on the dangers and benefits of nitrates
Everything is poison, the only question is the dose. (c) Paracelsus
What does the average person know - a person far from chemistry and physics - about nitrates (NO 3 - )? It is possible that this is a fertilizer, and concurrently a salt of nitric acid. Having pulled himself up, he will remember, perhaps, the safely forgotten course of school chemistry, when the “nitrogen”, especially fuming (more than 63-64%), burned everything in its path, dissolved gold in a mixture called “royal vodka” and denatured the proteins (yellow chemical skin burns - mmm, I love it!). Perhaps fans of healthy lifestyle will add that nitrates are carcinogens and poisons of the modern agricultural industry, and put them on a par with pesticides. And, actually, that's all ...
Such pictures on the device manufacturer’s website can scare anyone. Source
In fact, the nitrates themselves are quite harmless salts, well soluble in water. Unlike nitric acid, nitrates are not so strong oxidizing agents. Depending on the conditions (temperature, pH aka acidity of the medium, direction of reaction, etc.), nitrates have a standard redox potential of +500 to 900 mV, while really strong oxidizing agents, such as cerium (IV) oxide, have a standard potential of up to Ce 3+ +1610 mV - they can even oxidize water and decompose it into oxygen and H + (standard potential +1210 mV).
It turns out that nitrates are harmless ?!
From the point of view of the electrochemistry of aqueous solutions - yes. However, it is worth remembering that inside living organisms there are no aqueous solutions as such, and all reactions occur on the surface of membranes or inside special protein complexes called enzymes. Therefore, an answer that is close to reality: it all depends on which organism and concentration of nitrates we are considering.
Moreover, all carbon life on this planet is substantially energy surplus. In fact, all of us, all plants and animals, even the simplest microorganisms, are reservoirs of energy that exist only because of kinetic constraints. According to thermodynamics, we all should have burned long ago without a trace in an atmosphere containing 20 volume% oxygen. Accordingly, the presence of even such a weak oxidizing agent as nitrate, or its derivatives, can lead to undesirable redox reactions (OVR). Usually, the body has a protective mechanism - antioxidants , which help us not to “burn out” in the oxidizing environment of this planet.
And here we come to the paradoxical conclusion: it seems that nitrates are also harmful to eat (undesirable OVR, more on that below), but if there are more antioxidants, then, it seems, it's okay. Actually, this is why sausage nitrates and nitrites (stabilizers and preservatives) are much more harmful than vegetable from fresh vegetables and fruits, since in the latter the dose of antioxidants is significant.
Where do nitrates come from in plants?
Plants and bacteria that inhabit the soil often in symbiosis with the same plants participate in the giant cycle of the nitrogen cycle in nature. We will not dwell on it; more details can be found on Elementy.ru and on the Wiki .
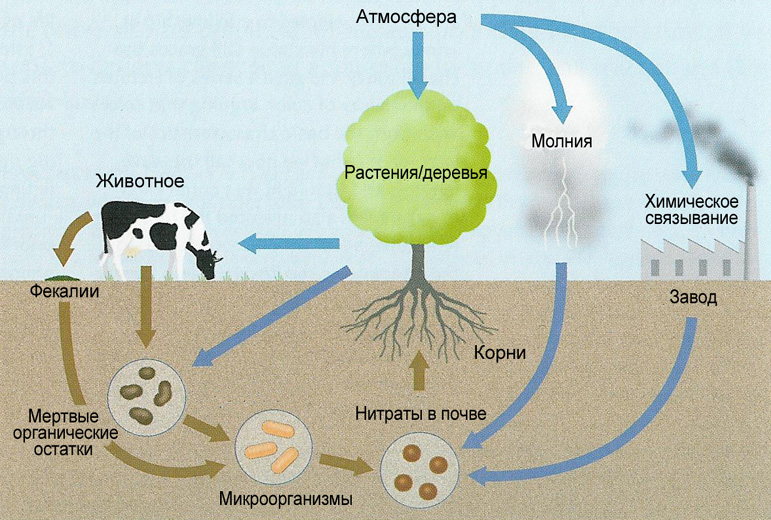
Extremely schematic representation of the nitrogen cycle in nature. Source
In the Earth’s atmosphere, 78 vol% is nitrogen, but atmospheric nitrogen is poorly absorbed by both bacteria and plants. Molecule N 2 is too inert. Only special bacteria that live in the earth can “digest” it. It is easy to guess that they are doing it extremely slowly. So slowly that depleted land is being restored for several years. Our ancient ancestors could tell a lot of interesting things about the constant deforestation for new lands, due to the permanent depletion of the soil and the associated crop failure.
However, there are other sources of bio-nitrogen - nitrate or the very nitrates that have excellent solubility in water - read, availability for cells and organisms. They only need to be restored from the oxidation state of 5+ (NO 3 - ) or 2+ (NO 2 - ) in the case of nitrites to 2- or 3- (amines). Of course, nitrite is slightly better than nitrate for plants, however, if we take into account that nitrite is an unstable form and over time it disproportionates to aka decomposes into NO 3 - and N 2 / NO, then nitrate is a gift from heaven for agriculture and your household flowers.
And then this bio-nitrogen is included in a long cycle until each atom is built into the molecule of the same chlorophyll based on porphyrin rings with four nitrogen, and, accordingly, is used for photosynthesis.
Since nitrates enter the plants through the soil, a direct conclusion suggests itself: there will be more nitrates in root crops than in fruits hanging on branches.
On the other hand, the earlier the fruit / vegetable, the more nitrates will be in it, since to get an early crop, plants are stimulated in every possible way, and the fruits are ripened unripe (read - with the maximum amount of nitrate from fertilizers). The more ripe the fruit / vegetable, the less nitrate in it, since most of them are "digested" by the plant itself, even if the plant is abundantly fertilized.
What happens to nitrates in the human body?
Nitrates through nitrites (NO 2 - ) can be converted to nitrosamines , which in high doses are fatal to animals. Nitrites are formed in our digestive tract from nitrates under the action of nitrate reductase - our body protects us from unwanted oxidants, as mentioned above. However, in such insignificant concentrations that it is more likely to do good than harm. Truly Paracelsus was right in his dictum.
Further, as our phytochemist steanlab wrote, nitrates and nitrites (the second more than the first) at high concentrations bind and actually “kill” heme-containing compounds, for example, the same hemoglobin, turning it into methemoglobin.
By the way, on the cyberleninke there is a good article about nitrates , which addresses the issue of the negative effects of nitrates and their chemical determination in various vegetables in 2017-2018.
And a few life hacks for paranoid nitrate:
- eat more vegetables that are high in antioxidants (lettuce, spinach, etc.),
- carry out heat treatment of products (more harmful nitrites will decompose),
- peel (peel is primarily a waste disposal system) and soak fruits / vegetables in water (ALL nitrates are highly soluble in water) before use.
So, we finished the theoretical part, get acquainted with the apparatus and look at the measurement results.
Experimental part
About the device used
As I noted above, there have been plenty of reviews and articles, including those of an advertising nature. Below I would like to understand in detail what is inside the device? Is he worth his money? and is there any secret chip in the device that measures nitrates?
Since this article is NOT an advertisement. The device was bought at my own expense (check available) for purely scientific purposes / interests. Therefore, a full description of the device, its claimed characteristics are hidden in the spoiler below.
The name of the device and the price
Today we will be tinkering around inside the Soeks F4 for 9900 rubles as of the summer of 2018. Now it costs a thousand more, although versions appeared without radiation and measuring electromagnetic radiation, simplified versions F2 and F3. By the way, on Aeroflot flights, its cost reaches 180 euros - a prohibitive price tag!
Amid the rampant bio-enthusiasm, Soeks is actively entering international markets. For example, like that .
There are already two certificates from the ecovisor - both certificates of conformity of the goods. In Russian: about nothing.
Here you can familiarize yourself with the errors, which are ~ 10-20%.
Amid the rampant bio-enthusiasm, Soeks is actively entering international markets. For example, like that .
There are already two certificates from the ecovisor - both certificates of conformity of the goods. In Russian: about nothing.
Here you can familiarize yourself with the errors, which are ~ 10-20%.
The device is delivered in a box with the necessary instructions, measurement brochures and charging.

Source
Since I like to delve inside the devices, let's take it apart and take a look inside. What is there such a company in store for us? For on the site it looks like a high-tech alien ship:
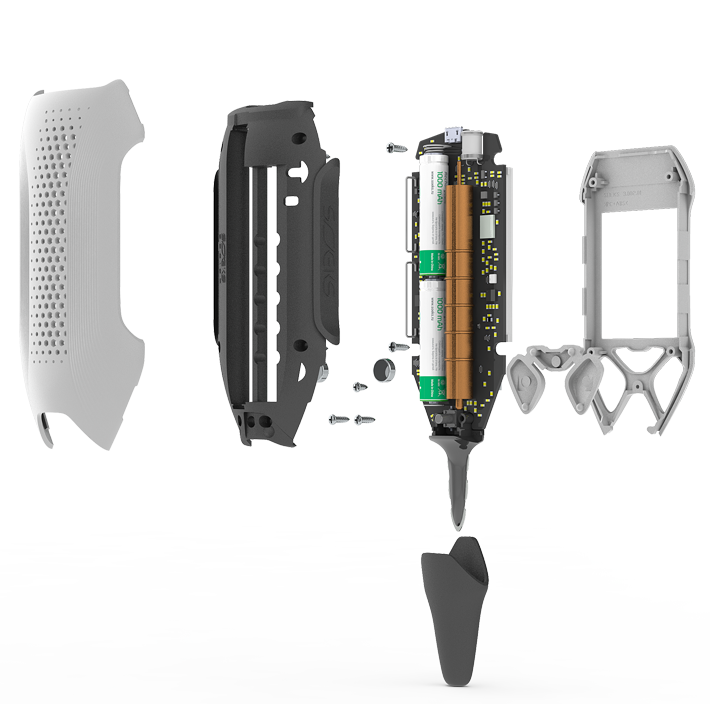
Source
Inside look
The full video with the analysis of this device is posted on my YouTube channel:
As you can see, the fantasy of the company's engineers (see above) and reality are somewhat different from each other. Case material - ABS with extremely mediocre casting. So that between the two halves of the case there are gaps in the thickness of the nail (recall, the cost of the device is ~ 10k rubles).
Let's go through the main components.
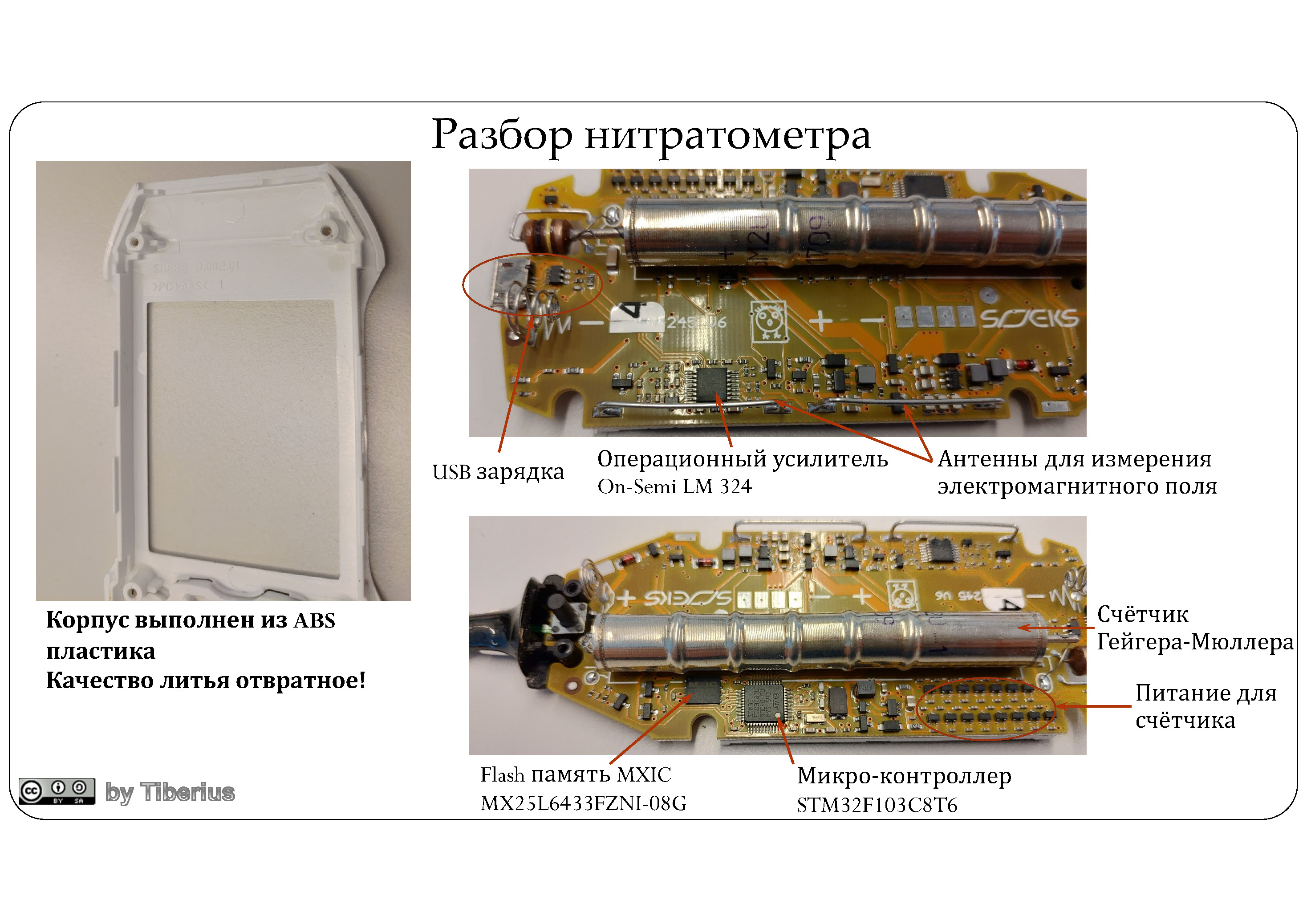
Inside, there are three large microcircuits waiting for us:
- microcontroller STM32F103C8T6 ,
- memory from MXIC aka Macronix MX25L6433FZNI-08G ,
- the operational amplifier aka operational amplifier (OP) is the LM 324 from ON-Semi (but this is not accurate).
It’s a sinful thing, I thought that OP stands for electrochemical measurements, however, if I don’t confuse anything, then OP works to receive radio waves through two arc antennas. Although, making a nano-potentiostat at home is not so difficult , and even based on the above OP .
Everything else is the power supply and stabilization circuits thereof. So, for example, to create the necessary voltage in the tube for measuring radiation fields, it took as many as 15 transistors (see counter power). Actually, here is a similar Geiger-Muller tube on Ali , which requires hundreds of volts for normal operation. How this tube works can be read here and here .
UPD: Buhram believes that these are not transistors, but simple diodes needed for the Cockroft-Walton generator power circuit.
In general, this device is an advanced conductometer with some chips (radiation + electromagnetic fields), which will respond to the presence of any ions. On the one hand, this is good - you can, for example, determine “pumped” or stale meat, on the other hand, the same chlorides / sulfates will affect the measurement results.
To make sure that this is really some kind of conductivity meter and extraneous ions really affect the readings, we will do the following test. Take a glass of water, measure “nitrates” in it. I got something about 15 mg . Yes, in plain tap water. And then add a spoonful of salt or a spoonful of sugar. In the first case, nitrates from 15 mg soared to 1300 mg (it seems to me that this is close to the threshold, although it is indicated as 5000 mg ), and in the second, they rose to only 30 mg . Salt aka NaCl is a strong electrolyte, which quickly dissolves into ions in solution, and glucose is weak and there are many neutral molecules with ions.
So how does the device work?
Very simple: they took "standard" samples of vegetables, measured the conductivity in them, perhaps then they measured the nitrates by a third-party method (although I doubt it). Ideally, in three, in near-scientific reality in two, in reality, a calibration was constructed at one point, which correlates the conductivity and the content of nitrates. All! Programmed and now sold as a product made in Russia.
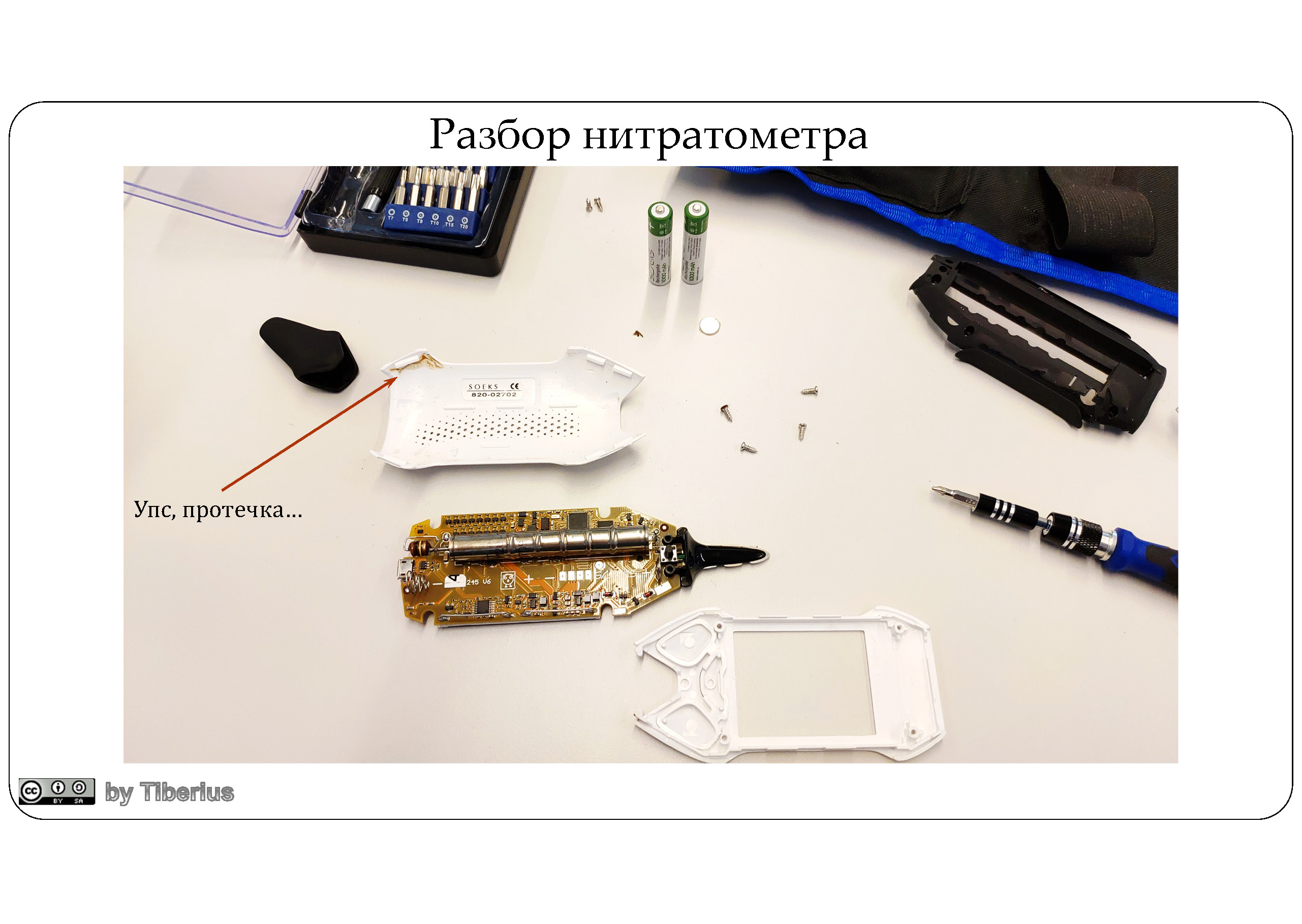
For a year of completely NOT intensive operation, the following problems were discovered:
- Battery - full slag. Although they are Ni-MH, they are apparently made at a Chinese noname factory with approximately the same quality. Within a couple of months, they began to die. Charging was enough for a couple of measurements, so the power-bank became a constant companion of the tester.
- A couple of times the display somehow died, that is, it seems that there is a backlight, but nothing is displayed. A couple of on / off cycles and it worked as it was. Now it feels like the screen has moved 2-3 lines up. Yes, by itself.
- A dozen products are irrelevant for our market. Well, where will you test durian or jack fruit?
- The case is leaky, which means that sooner or later the device will burn out, because after measurements it must be washed. There is not even partial protection against moisture.
- The temperature sensor died after 2 months and now shows a temperature from -100 to +100 C. It is not known whether the temperature is taken into account when measuring, whether it is corrected.
The result for the device is disappointing: it is absolutely not worth its money, but inside there is no “secret” device for measuring nitrates.
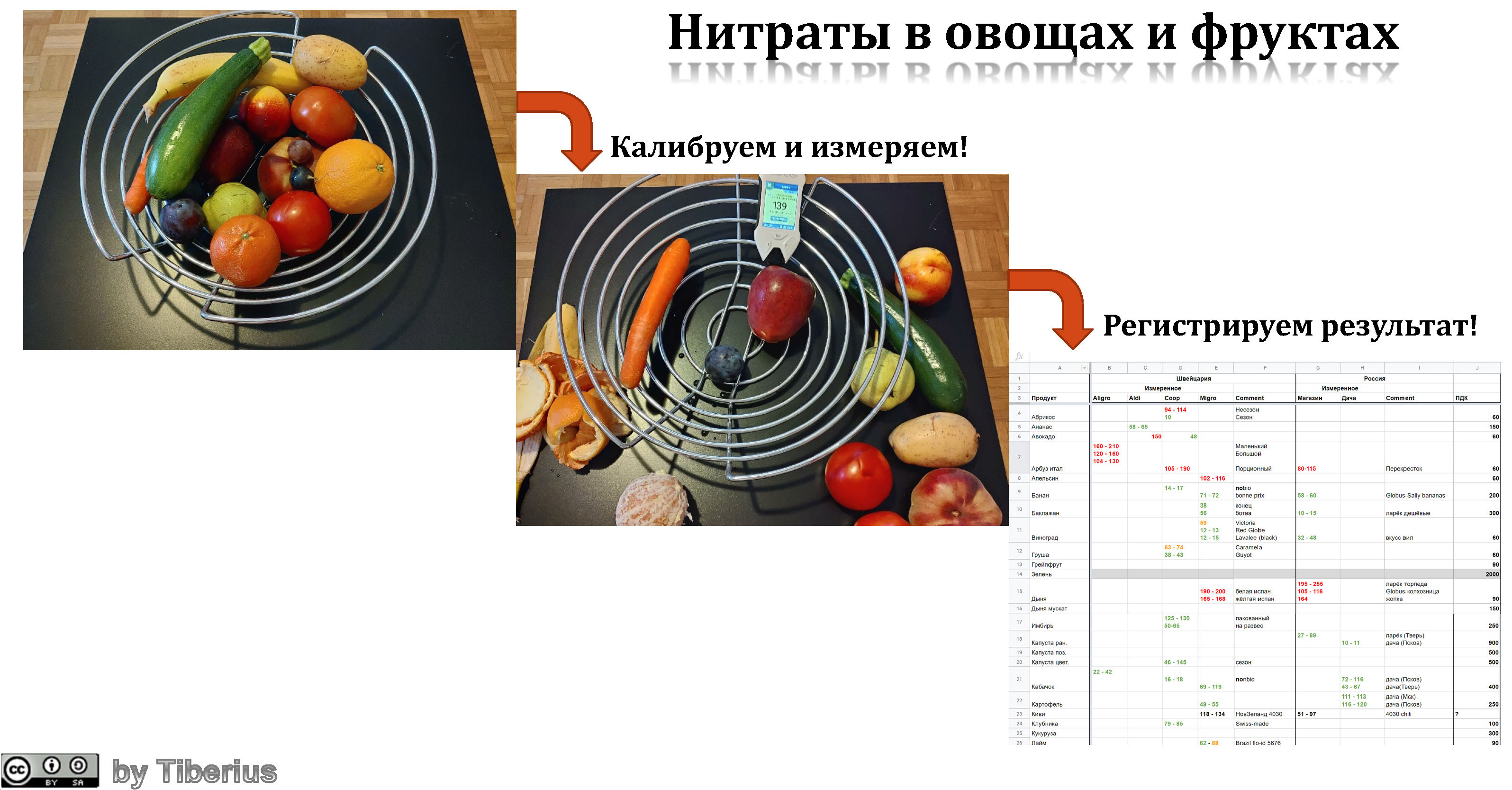
Measurement procedure
Well, we got acquainted with the internal device of the nitrate meter, which, to put it mildly, does not shine. Let's now move on to field tests and try to compare vegetables and fruits in Switzerland, Russia and with a Russian summer cottage.
I must say right away that this comparison is only of QUALITY character (~ 12-15% - this is the error declared by the manufacturer in ideal conditions.). The data obtained only say that this product is quite safe to eat or not. And for fruits with safe doses of nitrates up to 60 mg, it is practically useless.
All measurements are carried out within the following assumptions:
- We will assume that the concentration of ions other than nitrates is extremely small (at times). Nitrates and nitrites are better soluble than all other salts and may contribute to salting out .
- The difference between the fruits of one category is small. That is, if we measure greenhouse cucumbers, it does not matter which variety.
- The difference between batches of products is minimal. As will be seen from the table below, for the same product purchased in the same store, we have approximately the same data (see plum, watermelon, and a number of products measured twice).
So, for measurements, I chose 2-3 points where the probe stuck. One was closer to the "end" of the fetus, the other was closer to the stem, branch. The stem / branch should have the highest “nitrate” content, and at the opposite end the lowest, since all nitrate is “digested” by the plant.
Vegetable and fruit stores looked something like this:

All data collected is available here .
To summarize
Firstly, a surprise: products in Switzerland and Russia are not much different from each other in terms of the content of “nitrates”. What is called, within the margin of error. We have it big, it’s convenient to write off everything on it.
Secondly, practice shows that such categories of products as watermelons, melons, peaches and other imported fruits / vegetables are highly advisable to use EXCLUSIVELY in season. But even in the season after Italian nitrate watermelons, my gastrointestinal tract subtly hinted ... And God forbid, trying to look for strawberries in the winter, believe me, it will not bring benefits (solid growth stimulants) and pleasure (almost no smell).
Although the opposite example is given here , when the nitrate meter overestimates the performance of normal watermelons and melons and understates it of zucchini.
Let me give you another example, all agricultural products in Switzerland are strictly seasonal:
- cherries / cherries (almost no cherries, by the way, grows wild, but nobody needs it) - June-July;
- apricots, plantations which are full of the Rhone Valley in the Valais, are consumed from July to September;
- grapes on shelves in huge quantities and at nice prices appear only in late August and until the end of September, occasionally affecting October;
- about asparagus, the cult of which is simply forbidden here, I am silent - exclusively May / June;
- persimmon with the sonorous name kaki - October / November;
- tangerines / oranges - from November to the New Year holidays.
Just some products are replaced by others.
Thirdly, there is indirect evidence that “buttocks” and “assholes” have a higher concentration of nitrates than the rest of the fetus.
Fourth ...
About the cottage
Surprisingly, it’s almost a fact: country products are really more “eco” and “bio” than from the store. All measured products from 3 different cottages were surprisingly clean.
However, the cottage is different. For example, if every year the earth is poured with nitrate, then the products will be with a high content of nitrates. If fertilizers and manure are left with humus for 1-2 seasons, and only then used as fertilizing for plants, then the products will be perfectly clean, as it is now fashionable to say, bio ...

Unfortunately, I could not find the link, but somewhere there was an article that suburban products were 7 times more expensive than store ones. Maybe tell me?
Conclusion
If you have extra 10k, then
If this topic arouses public interest (likes / views), then you will have to start an annual experiment, where for the selected product categories, for example, apples, tomatoes, cucumbers, zucchini, make an annual cut and see how the amount of “nitrates” in them changes during the year .
Bonus
To the question of the latest radiation hysteria.
We measure the background radiation in flight over old Europe. In the same table in google documents, but in the next sheet data on altitude radiation are given.
I used the EMU measurement function only a couple of times: with a router, a mobile phone and other emitters - yes, it is better not to sleep in an embrace.
Do not forget to subscribe to the blog : it’s not difficult for you - I am pleased!
And yes, please write about the shortcomings noted in the text in the PM.
Special thanks to berez for proofreading the material!
All Articles