Work on it by Alan Saghatelian et al. was published in Nature Communications.
The study began when scientists discovered PIGBOS in mitochondria.
The first attempt to detect PIGBOS protein through green fluorescent protein (GFP) failed. Microprotein was too small compared to the size of GFP. The team of scientists solved this problem by trying a less common approach called shared GFP, in which they combined only a small part of the GFP, called the beta, with PIGBOS.
Researchers were able to see PIGBOS and study how it interacts with other proteins. When they mapped the location of PIGBOS, they realized that it was on the outer mitochondrial membrane, ready to make contact with proteins on other organelles. They were surprised to see that PIGBOS interacts with a protein called CLCC1, which is part of an organelle called the endoplasmic reticulum (ER).
“PIGBOS serves as the junction between mitochondria and ER,” says another co-author of the work, Qian Chu. "Before, we did not see this in microteins - and rarely found in ordinary proteins."
Researchers found that PIGBOS actually binds to CLCC1 to regulate stress in ER. Without PIGBOS ER, it is likely to experience stress, which leads to a chain of events when the cell tries to clean out the deformed deformed proteins (response to improper protein folding, UPR). If a cell cannot get rid of these proteins, it initiates a self-destruction sequence and dies.
Scientists did not expect to see the role of mitochondrial protein in the UPR reaction. This new understanding of PIGBOS opens the door to future treatments that can target stress cells.
“In the future, we could consider how PIGBOS is involved in diseases like cancer,” says Q. Chu. "In patients with cancer, ER is more stressful than in a normal person, so managing stress ER can be a good goal."
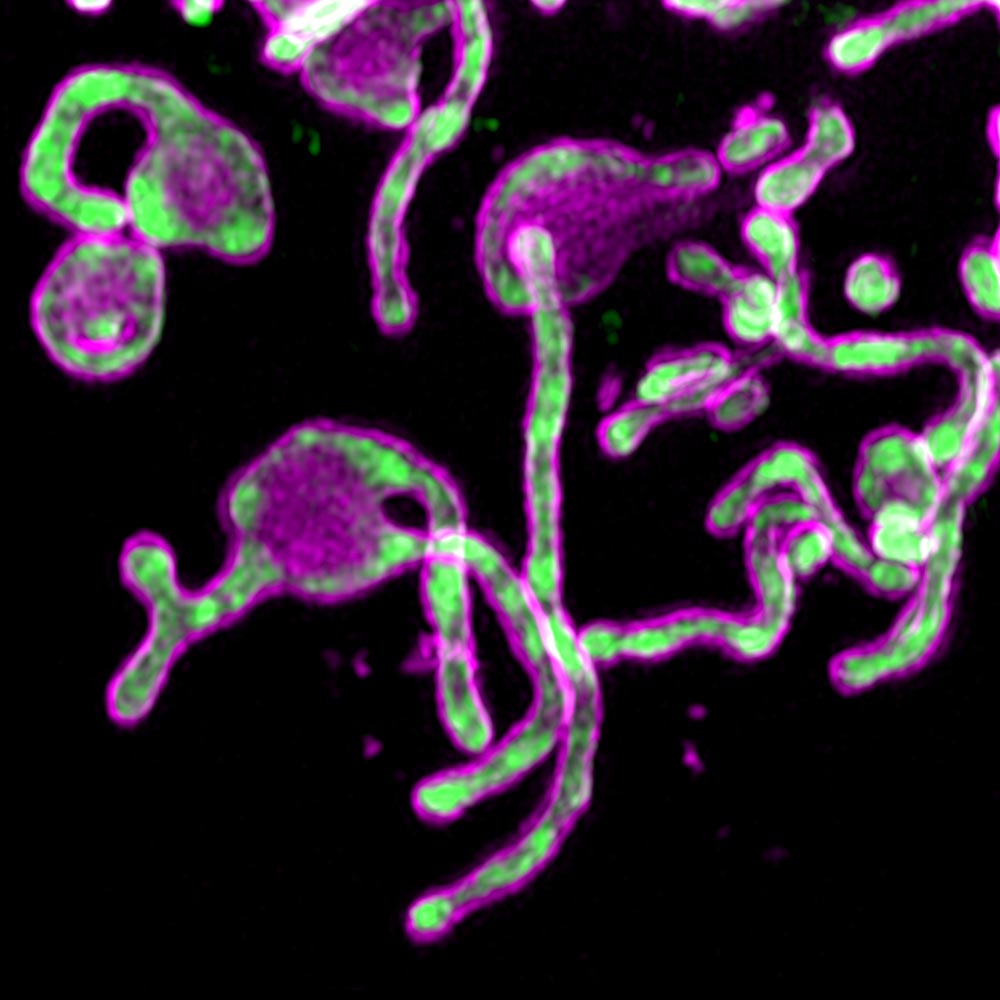
Fig. 1. Visualization of the microprotein PIGBOS
www.salk.edu/news-release/mysterious-microproteins-have-major-implications-for-human-disease
Researchers are interested in studying the role of other mitochondrial proteins in ER stress, as well as how PIGBOS works in animal models. The team is also moving forward in characterizing an extensive library of microteins that can be critical in cell biology.
What are microproteins?
The term "microteins" refers to small proteins that are encoded by small open reading frames (smORF). Advances in genomics and proteomics technologies show that mammalian genomes are thought to contain hundreds to thousands of microteins encoded by smORFs. As a large and still poorly studied part of the genome, microteins provide a great opportunity to gain a new understanding of modern biology.
It is no coincidence that the American journal The Scientist called microproteins "the dark matter of the human proteome." Although the first of them, Id, was discovered about 30 years ago. A common characteristic of the family of Id proteins in mammals is their regulation of cell fate. Id proteins act in various tissues and cells, including myoblasts, the nervous system, and the immune system. Besides the fact that they act as modulators of the transcription mechanism and affect the fate of developmental cells, Id proteins also affect cell cycle control and are overexpressed in various human tumors. The molecular function of Id proteins in developmental processes is well understood, but little is known so far about the function of Id proteins in adults.
To date, only a few smORFs and microteins are well characterized. For example, some muscle-specific smORFs have allowed us to describe new pathways that control muscle function and development. MOTS-C human microprotein regulates metabolic homeostasis, NoBody microprotein (non-annotated P-body dissociating polypeptide) interacts with mRNA cleaving proteins, which are the molecular components of the first enzymatic step in the mRNA breakdown pathway. A microtein called CYREN regulates the choice of DNA repair pathway during the cell cycle.
The currently known mechanism of action of microproteins is to suppress the formation of protein complexes. And this is what it looks like. Many proteins fulfill their functions by acting as part of multi-protein complexes. The formation of these complexes is strictly regulated and mediated by protein-protein interaction domains. Disruption of the complex or ability of proteins to form homodimers, heterodimers, or multimers can have serious consequences for cellular function. In this regard, the formation of dimers and multimers can be disrupted by microproteins. Microproteins behave as post-translational regulators, forming homotypic dimers with their targets, and act through dominant-negative suppression of the function of the protein complex.
Simply put, microproteins interfere with the complex work of larger proteins, inhibiting some cellular processes and stimulating others. Studies show that the action of microteins is evolutionarily conservative and common to both the animal and plant kingdoms. The results of primary studies show that microproteins are involved in immune processes, control the destruction of defective RNA molecules, protect bacteria from heat and cold, dictate the flowering period of plants, and serve as a source of toxins of many types of animal poisons. According to scientists, it seems that microproteins are involved in all biological processes. Just before they did not pay attention.
It is likely that many other key cellular processes are also mediated by undescribed microteins. Both the detection and characterization of smORFs and microteins are an important research task.
Despite the remaining mysteries, scientists are already testing the potential use of these molecules. One company sells insecticides derived from microproteins found in the poison of an Australian atracide. In clinical trials, a contrast agent based on another tiny protein in scorpion venom is tested. The task of this substance is to highlight the boundaries of tumors so that surgeons can extract them with greater accuracy. Many pharmaceutical companies are now looking for micro-proteins with medical potential.
How tiny they can be is still unclear. Drosophila need a microprotein with 11 amino acids to grow normal paws, and some microbes can produce proteins less than ten amino acids in length. But even the largest microteins do not reach medium-sized proteins, such as alpha-amylase, a 496-amino acid enzyme in our saliva that breaks down starch.
Only the recent detection of a small amount of microteins is associated with the gene recognition criterion established about 20 years ago. When scientists analyze the body’s genome, they often scan an open reading frame (OPC). In order not to drown in a huge amount of data, in the past, researchers generally ruled out any ORS with a protein of less than 100 amino acids in eukaryotes or 50 amino acids in bacteria. For example, in yeast, this condition limited the list of OPCs to about six thousand.
The weakening of this criterion shows that the cells contain significantly more OPC. Earlier this year, scientists identified fragments of the genome of microbes that inhabit four parts of the human body, including the intestines and skin. When searching for small OPCs that can encode proteins between five and 50 amino acids long, the researchers identified about four thousand families of potential microteins. Almost half of them are not similar to known proteins, but the sequence of one small ORS suggests that the corresponding protein is located in the ribosomes - and this indicates that it can play some fundamental role. When scientists overlooked small OPCs, not just genes with some rare functions were missed, but genes with key functions.
Other cells also contain a huge number of short OPCs: yeast, for example, is able to produce more than 260 thousand molecules with a spectrum from two to 99 amino acids. But cells almost certainly do not use all of these ORSs, and some of the amino acid chains they produce may not be functional.
Weissman et al. found microproteins in another way: using the method they invented, aimed at more widespread identification of what kind of proteins are produced by cells. To form any protein, the cell first copies the gene into an RNA messenger. Then the ribosomes read the mRNA and bind the amino acids in a specific order. By sequencing the mRNA attached to the ribosomes, Weissman and his team accurately determine which ones actually turn into proteins, and where the ribosome begins to “read” on the RNA. In a 2011 cell study, a scientist with his team applied this ribosome profiling method, also called Ribo-seq, to mouse embryonic stem cells and found that the cells produce thousands of unforeseen proteins, including many in which the number of amino acids will be below the threshold of 100 units. “It was very clear that the vast universe of proteins, many of which were short, was ignored as part of the standard approach,” says Weissman.
Saghatelian and colleagues took a third approach to detect the abundance of microteins in our own cells. Scientists used mass spectrometric analysis, in which the proteins are split into fragments sorted according to mass, in order to identify the identification spectrum of each protein. Saghatelyan and colleagues applied this method to mixtures of proteins from human cells, and then subtracted from them the signs of known types of proteins. This method revealed spectra of 86 previously unknown tiny proteins, the smallest of which were 18 amino acids long, as scientists noted in an article in the journal Nature Chemical Biology in 2013.
Small size limits protein potential. Larger proteins combine into complex forms aimed at the implementation of certain functions, for example, catalyzing chemical reactions. Proteins containing from 50 to 60 amino acids are not likely to form compounds. And therefore, they are not suitable for the formation of enzymes or structural proteins.
However, their small size also opens up certain possibilities. They are tiny enough to fit into the nooks of large proteins that function as channels and receptors. Small proteins often share short stretches of amino acids with their larger partners and therefore can bind and alter the activity of these proteins. Bound microproteins can also direct large molecules to new places - for example, by facilitating their penetration into cell membranes.
Because of their attraction to larger proteins, smaller proteins can give cells a reversible way of activating or deactivating larger proteins. In a 2016 study published in the journal PLOS Genetics, Stephan Wenkel and colleagues genetically modified Arabidopsis plants to produce an additional amount of two small proteins. Plants usually bloom at a time when the day is long enough, but after the birth of an excessive amount of two types of microteins, flowering was delayed. Small proteins provoked this delay by blocking the flowering large protein called CONSTANS. They bind CONSTANS to other inhibitory proteins that turn it off.
In 2016, Saghatelyan and his colleagues found that human cells produce a protein containing 68 amino acids, which they called NoBody. This protein can help with the destruction of defective or unnecessary RNA molecules. The name "Nikto" reflects the role of this protein in preventing the formation of processive bodies, mysterious clusters in the cytoplasm, where RNA destruction can occur. When there is not enough protein, more processive bodies are formed, which accelerates the destruction of RNA and changes the internal structure of the cell. “This proves that small proteins can have a huge effect inside the cell,” scientists say.
Muscles depend on many different microproteins. During the development of the embryo, individual muscle cells merge into fibers that trigger contraction. Myomixer 84-amino acid protein combines with a larger protein to connect cells, as Olson et al. Showed in 2017 in his work in the journal Science. Without it, mouse embryos cannot form muscles and are almost transparent.
Further, as it develops, myoregulin enters into force, which helps regulate muscle activity. When a muscle receives a stimulus, the cell storehouse releases calcium, stimulating tissue contraction and energy production. Next, an ion pump, a protein called SERCA, begins to return calcium to storage, allowing muscle tissue to relax. Mioregulin binds to SERCA and inhibits it. This effect limits the frequency of muscle contraction, probably providing an energy reserve for an emergency, for example, when fleeing from a predator. Another small protein, DWORF, has the opposite effect of activating SERCA and stimulating systematic muscle contraction.
Even thoroughly studied organisms, such as E. coli bacteria (Escherichia coli) hide unexpected small proteins that play an important role. Storz et al. reported in 2012 that a previously unknown 49-amino acid protein called AcrZ helps this microbe survive when exposed to certain types of antibiotics by stimulating a drug-free pump.
the venom produced by a variety of organisms - including spiders, scolopendras and poisonous mollusks - is also teeming with tiny proteins. Many components of the poison immobilize or kill, blocking the channels for sodium or other ions necessary for the transmission of nerve impulses. Small proteins "strike these ion channels with amazing accuracy and power, scientists say. They are the main components of poisons, and it is with them that most of the pharmacological and biological consequences are associated.
The giant fish-eating Australian insect, for example, does not just rely on sharp jaws and peak-like mouth parts to defeat its prey. It introduces to its victims a dose containing more than 130 proteins, of which 15 are less than 100 amino acids.
Unlike large proteins, such as antibodies, microproteins that are ingested from tablets or injections can penetrate cells and modify their functions. Captopril, the first of a class of high-pressure drugs known as an angiotensin-converting enzyme inhibitor, was developed from the small protein found in the common poison of heatworm, a species of poisonous pit viper. This medicine, approved for sale in the United States in 1981, was discovered by chance, even before scientists recognized small proteins as a separate group. So far, only a few microteins have hit the market or in clinical trials.
Cancer researchers are trying to use the microprotein found in yellow scorpion venom common in Africa and the Middle East. This molecule is mysteriously attracted to tumors. By adding it to a fluorescent staining substance, scientists expect to highlight the boundaries of brain tumors so that surgeons can safely excise areas affected by cancer .. It illuminates the tumor. Thus, you can see its boundaries and identify the presence of metastases. In clinical trials, researchers are now evaluating whether a dual molecule will help surgeons remove brain tumors in children.
How important small proteins will play in medicine is still unknown, but they have already turned over a number of biologists' claims. Norbert Hübner of the Max Delbrück Center for Molecular Medicine in Berlin and colleagues found dozens of new microteins in human heart cells. The group revealed their unexpected source: short sequences within long non-coding RNAs, that is, in a species that was previously thought to not produce proteins. By identifying 169 long non-coding RNAs, probably read by ribosomes, Huybner and his team used one of the types of mass spectrometry to confirm that more than half of them produce microproteins in heart cells. their performance paper was published this year in Cell.
The DNA sequences of other tiny proteins can also be found in unconventional places. For example, some are in open reading frames, OPC, larger proteins. Scientists previously believed that these sequences help control the production of larger proteins, but rarely produce proteins themselves. Some coding sequences of newly detected microteins lie even in sequences coding for other, longer proteins.
These genomic surprises can shed light on how new genes come about, says evolutionary systems biologist Anne-Ruxandra Carvunis of the University of Pittsburgh, Pennsylvania. Scientists previously believed that most genes appear when existing genes multiply or fuse, or when species exchange DNA. However, according to Karvunis, microproteins indicate that protogens can form when mutations set a new start and stop signals in the non-coding part of the genome. If the resulting OPC produces a useful protein, the new sequences will remain in the genome and go through the process of natural selection, further evolving into larger genes encoding more complex proteins.
In a 2012 study, Carvounis and colleagues found that yeast transforms more than a thousand short ORS into proteins. And this suggests that these sequences are protogens. In a new study, Carvounis and her team tested whether young ORS could be beneficial for cells. They genetically altered the yeast in order to increase the result of 285 newly evolving ORSs, most of which target smaller molecules than standard proteins or a little more than that. In almost 10% of proteins, an increase in their levels enhanced cell growth in at least one medium.
“Microproteins are a rapidly growing area,” says A. Saghatelian. “But I think that this study of ours really influenced the current understanding of the influence of microproteins on biochemistry and cell biology.”
References :
Regulation of protein function by 'microProteins'
New universe of miniproteins is upending cell biology and genetics
Regulation of the ER stress response by a mitochondrial microprotein