Died Nobel Laureate Carey Mullis - inventor of the polymerase DNA chain reaction
 In California, at the age of 74, American Nobel Laureate in Chemistry Carey Mullis died. According to his wife, death occurred on August 7. The reason is heart and respiratory failure due to pneumonia.
In California, at the age of 74, American Nobel Laureate in Chemistry Carey Mullis died. According to his wife, death occurred on August 7. The reason is heart and respiratory failure due to pneumonia.
James Watson, the discoverer of the DNA molecule, will tell us about his contribution to biochemistry and for what he received the Nobel Prize.
Excerpt from the book of James Watson, Andrew Berry, Kevin Davis
DNA The history of the genetic revolution
Chapter 7. The human genome. Life script
...
Polymerase chain reaction (PCR) was invented in 1983 by biochemist Cary Mullis, who worked at Cetus. The discovery of this reaction was quite remarkable. Mullis later recalled: “Once on a Friday night in April 1983, it seemed to me that it dawned on me. I was driving, rolling along a moonlit winding mountain road to Northern California, the edge of redwood forests. ” It is impressive that it was in such a situation that he was visited by inspiration. And it’s not at all that in northern California there are special roads that promote insight; it was just that his friend once saw Mullis rushing recklessly along an icy two-way road and this did not bother him at all. A friend told the New York Times the following: “Mullis dreamed that he would die by crashing into sequoia. Therefore, he is not afraid of driving anything if redwoods do not grow along the road. ” The presence of sequoia along the road made Mullis concentrate and ... here it is, an insight. For his invention in 1993, Mullis received the Nobel Prize in chemistry and has since become even stranger in his actions. For example, he is a supporter of the revisionist theory that AIDS is not related to HIV, which significantly undermined its own reputation and prevented doctors.
PCR is a fairly simple reaction. To carry it out, we need two chemically synthesized primers complementary to the opposite ends of the different chains of the desired DNA fragment. Primers are short sections of single-stranded DNA, each approximately 20 base pairs long. The peculiarity of the primers is that they correspond to the regions of DNA that you want to amplify, that is, the DNA template.

(Image clickable) Cary Mullis, inventor of PCR
The specificity of PCR is based on the formation of complementary complexes between the matrix and primers, short synthetic oligonucleotides. Each of the primers is complementary to one of the chains of the double-stranded matrix and limits the beginning and end of the amplified region. In fact, the resulting “matrix” is a whole genome, and our goal is to isolate fragments of interest to us from it. For this, the double-stranded DNA template is heated to 95 ° C for several minutes so that the DNA chains are dispersed. This stage is called denaturation, since the hydrogen bonds between the two DNA chains are destroyed. When the chains are open, the temperature is lowered so that the primers can contact the single chain template. DNA polymerase begins DNA replication by binding to a length of a nucleotide chain. The DNA polymerase enzyme replicates the template chain using a primer as a seed or copy example. As a result of the first cycle, we obtain multiple sequential doubling of a specific DNA region. Next we repeat this procedure. After each cycle, we obtain the target area in double quantity. After twenty-five PCR cycles (that is, in less than two hours), we have the DNA region of interest in the amount that is 225 times the original (that is, we amplified it by about 34 million times). In fact, at the entrance we got a mixture of primers, template DNA, DNA polymerase enzyme and free bases A, C, G and T, the amount of specific reaction product (limited by primers) grows exponentially, and the number of “long” DNA copies is linear, therefore reaction products dominate.

Amplification of a desired DNA site: polymerase chain reaction
At the dawn of PCR, the main problem was as follows: after each heating-cooling cycle, DNA polymerase had to be added to the reaction mixture, since it was inactivated at 95 ° C. Therefore, it was necessary to re-add it before each of the 25 cycles. The reaction procedure was relatively ineffective, it required a lot of time and the polymerase enzyme, and the material is very expensive. Fortunately, Mother Nature came to the rescue. Many animals feel comfortable at temperatures well above 37 ° C. And why did the figure 37 ° C become important for us? This happened because this temperature is optimal for E. coli, from which the polymerase enzyme for PCR was originally obtained. In nature, there are microorganisms whose proteins have become more resistant to high temperatures over millions of years of natural selection. It has been proposed to use DNA polymerases from thermophilic bacteria. These enzymes were thermostable and were able to withstand many reaction cycles. Their use made it possible to simplify and automate PCR. One of the first thermostable DNA polymerases was isolated from the Thermus aquaticus bacterium that lives in the hot springs of Yellowstone National Park and is called Taq polymerase.
PCR quickly became the main workhorse of the Human Genome project. In general, the process does not differ from that developed by Mullis, it was just automated. We no longer depended on a crowd of blind-eyed graduate students painstakingly pouring droplets of liquid into plastic tubes. In modern laboratories carrying out molecular genetic research, this work is carried out on robotic conveyors. PCR robots involved in such a large-scale sequencing project as the Human Genome are inexorably working with huge volumes of heat-resistant polymerase. Some scientists working in the Human Genome project were outraged by the unjustifiably high contributions that the PCR patent holder, the European industrial and pharmaceutical giant Hoffmann-LaRoche, adds to the cost of consumables.
Another “driving force” was the DNA sequencing method itself. The chemical basis of this method at that time was no longer new: the Interstate Project Human Genome (HGP) adopted the same ingenious method that Fred Senger developed in the mid-1970s. The innovation was in the scale and degree of automation that was achieved during sequencing.
Automatic sequencing was originally developed at Lee Hood's laboratory at the California Institute of Technology. He graduated from high school in Montana and played American football as a striker; thanks to Hood, the team has won the state championship more than once. Teamwork skills came in handy for him in his scientific career. Hood's lab employed a motley company of chemists, biologists, and engineers, and soon his laboratory became a leader in technological innovation.
In fact, the method of automatic sequencing was invented by Lloyd Smith and Mike Hunkapiller. Mike Hunkapiller, who then worked in Hood's laboratory, turned to Lloyd Smith, offering him an improved sequencing method in which the bases of each type would be painted each in its own color. Such an idea could quadruple the effectiveness of the Senger process. Sanger, when sequencing in each of the four tubes (according to the number of bases) with the participation of DNA polymerase, forms a unique set of oligonucleotides of different lengths, including a primer sequence. Next, formamide was added to the tubes to separate the chains and polyacrylamide gel electrophoresis was performed on four tracks. In the Smith and Hunkapiller variant, the dideoxynucleotides are labeled with four different dyes and PCR is performed in one tube. Then, during polyacrylamide gel electrophoresis, a laser beam at a specific location in the gel excites dye activity, and the detector determines which nucleotide is currently migrating through the gel. At first, Smith was pessimistic - he was afraid that the use of ultra-low doses of the dye would lead to the fact that the nucleotide sites would be indistinguishable. However, being well versed in laser technologies, he soon found a way out using special fluorochrome dyes that fluoresce under the influence of laser radiation.
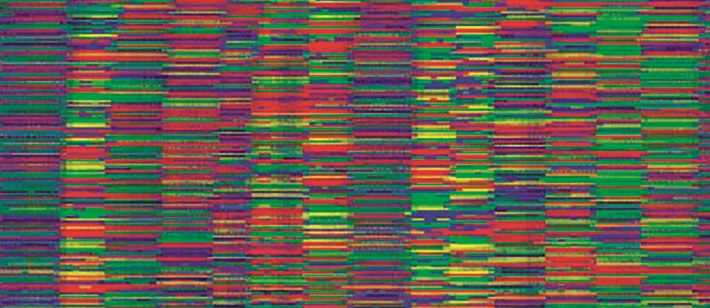
(Full version by click - 4.08 MB) Small print: DNA sequence decoded using an automatic sequencer obtained from an automatic sequencing machine. Each color has one of four bases.
In the classical version of the Sanger method, one of the strands of the analyzed DNA acts as a matrix for the synthesis of a complementary strand by the enzyme DNA polymerase, then the sequence of DNA fragments is sorted by size in a gel. Each fragment that is included in the DNA during synthesis and subsequently allows visualization of reaction products is marked with a fluorescent dye corresponding to the terminal base (this was discussed on page 124); therefore, the fluorescence of this fragment will be an identifier for this base. Then it remains only to carry out the detection and visualize the reaction products. The results are analyzed using a computer and presented as a sequence of multi-colored peaks corresponding to four nucleotides. Further, the information is transmitted directly to the computer information system, which eliminates the time-consuming and sometimes painful data entry process, which greatly complicated sequencing.
»More details on the book can be found on the publisher’s website
» Contents
» Excerpt
For Khabrozhiteley 25% discount on the coupon - PCR
All Articles