Guide to electrical materials for all. Part 4
Continuing guidance on electrical materials. In this part we begin to disassemble dielectrics, the part is completely devoted to inorganic dielectrics: porcelain, glass, mica, ceramics, asbestos, SF6 gas and water.

Welcome to CAT (TRAFFIC)
In addition to conductors, dielectrics are needed for the production of electronics. Depending on the conditions and tasks, different properties of the dielectric may be important: heat resistance, loss tangent, hygroscopicity, mechanical strength, etc.
The manual section with polymers is even more superficial. The fact is that the properties of the polymer material depend on the synthesis conditions, the additives introduced, the heat treatment, and the subsequent processing. Thus, two samples of polystyrene can vary significantly in properties. Manufacturers of plastics go to various tricks and manipulations with the composition, making important and not very changes. It's like with books, different editions of the same work, somewhere on a newsprint with a bad layout, and somewhere on a quality paper with color illustrations from a fashion artist. Both books are “Lord of the Rings”, but impressions of use may differ. Therefore, some general properties of different types of polymers are given; for more precise characteristics, refer to the reference book.
Materials that are used in electronic technology change as progress progresses. So, earlier it was widely used, for example, wood, silk, ebonite. Today, many materials have been superseded by cheaper, more technologically advanced substitutes. The manual has a description including historical materials, data for general development. Also added the information necessary for the completeness of the disclosure of the topic.
Porcelain is dense solid ceramics obtained by burning a mixture of kaolin, quartz, feldspar and clay. It is similar to a porcelain cup in your kitchen, only less often it is coated with icing.
High temperature insulators. In the form of porcelain beads to isolate the ends of the heating coils. The scaly-like construction allows for flexing without exposing the conductor.

The case of a mercury arc lamp from a light beam oscilloscope. Aluminum alloy frame, black body - carbolite, porcelain beads isolate the conductors that connect the lamp. The lamp is very hot during operation. A bunch of porcelain beads from various heaters.

Spark plugs from an internal combustion engine. The central electrode is insulated by porcelain. No other dielectric is able to withstand prolonged exposure to temperature, pressure, fuel inside the combustion chamber.
Details of electrical products. If you look inside the lamp holder, the part that contains the connecting lamellae is most likely made of porcelain, it can work for a long time at elevated temperature of the incandescent lamp without losing properties. Cases of fuses, sockets, holders of contacts of lamps - wherever there is a danger of heating, china is out of competition.

Holders of lamellae sockets, cartridge made of porcelain. The black body of ammunition - carbolite.

Power resistors have a porcelain tube base. The green resistor winding is hidden under the enamel.
Insulators on poles. In the photo there is an isolator from a pole that was liquidated during the reconstruction of the line. 30 years of the sun, wind, bird droppings, rain, frosts did not affect the porcelain, it still looks like new, it was enough to wash the insulator with soap.

Porcelain insulators of power lines. Between the porcelain insulator and the steel hook there is a polyethylene bush, to protect the porcelain from cracks. The disk form of the insulators allows water to flow down without forming a continuous layer that closes the conductor onto the support.
Fragile, like all ceramics. Tightened screw, blow - and porcelain showered.
Depending on the requirements, different types of glass can be used, from low-melting sodium to refractory quartz. The main plus of glass, in addition to its heat resistance, is transparency for visible light (and quartz glass is also transparent for ultraviolet). Also an important plus is the ability to visually assess the integrity, cracks are usually visible.
Enclosures of radio tubes, lighting lamps, fuses. Quartz tubes - cases of heaters, electric grills.

Glass and porcelain insulator power lines working on the street for over 30 years.
Fragile, does not take blows. Some types of glass crack with sudden uneven heating.
A typical feature (but not obligatory!) Of quartz glass is a large number of cores in the direction of glass extrusion.
Here it is worthwhile to additionally say about sapphire glass, tempered glass and chemically tempered glass. In the advertising descriptions of many electronic devices for mass consumption can be found references to these types of glasses.
Mica. Natural layered material, has heat resistance, durability, excellent dielectric. Mica is a large class of layered minerals, of which muscovite is used mainly in technology and sometimes biotite and phlogopite.
In English, mica is Mica, hence derived names of materials based on mica - micanites, mikalenta, micafolium, mikaleks, etc.
Mica mined in the mine is disassembled, sorted. Large pieces are manually split into plates - this is plucked mica - transparent homogeneous plates. Such mica has the highest quality and is used for critical applications - in vacuum technology, radiation input / output windows, etc. Unfortunately, large homogeneous pieces of mica without defects are a rarity, therefore mica plates of various shapes are glued together, so it turns out micanite . If a cloth (fiberglass, paper) is used as a substrate for sticking mica plates , micalenta, micafolium, and glass mikanit will be obtained . Minor mica wastes are ground, and in the form of water slurry, they are cast onto the net, just like paper. After removing water, the particles of mica stick together in a single canvas - it turns out mica paper (mica, mica) . The resulting fabric for strength can be impregnated with an organic binder. The flexibility of mica paper allows it to be wound as insulation. Also winding can be obtained rods, tubes. If mica is impregnated with molten glass, the resulting durable material is called miklex .
Ground into mica dust - a component of pigments, thanks to its “scales” gives a pearl effect. Biotite is mainly used in pigments.
Synthetic material - fluoroflogopite (synthetic mica) is mica (phlogopite) where -OH groups are replaced by fluorine. Fluoroflogopit is more durable and thermally stable, looks also like mica, also layered but absolutely transparent / white, and not yellowish, like natural mica. Alas, as long as I did not come across this material.
Structural elements for holding heating elements in hair dryers, heaters, fan heaters, soldering irons, etc.

Domestic fan heaters. The construction on the left is less material-intensive, but much less reliable, especially under mechanical loads.
As a protective window of the microwave radiation from the magnetron in the microwave. (Usually, when food gets mica, it becomes charred, and as a conductor, it starts to sparkle violently, which causes the microwave owners to throw out the microwave with fear, although it is enough to cut the mica out of the sheet and replace the window.)

Window output microwave radiation from mica.
Due to the fact that thin plates of mica do not let in gases, but they let in energetic charged particles - mica windows are used in the construction of alpha and beta particles counters.
Used in the construction of radio tubes - keeps the electrodes in place.

The octagonal plate is made of mica.
Used as mica capacitor material. Mica acts as a dielectric, and electrodes act as conductive metal deposition on mica plates. This type of capacitor is becoming less and less, replaced by capacitors based on polymer films. Mica capacitors can operate at high temperatures.

Mica capacitors produced in the USSR half a century ago.

Mica plates in the condenser. Metallization on the plates forms the plates.
Before the advent and widespread use of thermally conductive insulating gaskets made of polymeric materials like Nomakon, mica plates were used to electrically insulate the components while maintaining thermal contact, for example, when several transistors with different voltages needed to be attached to one radiator.

Records of natural plucked mica.
Earlier, several centuries ago, when they did not know how to make thin window glass, translucent structures made splitting natural mica. Since large pieces of mica without defects were rare, the windows took on a bizarre shape.
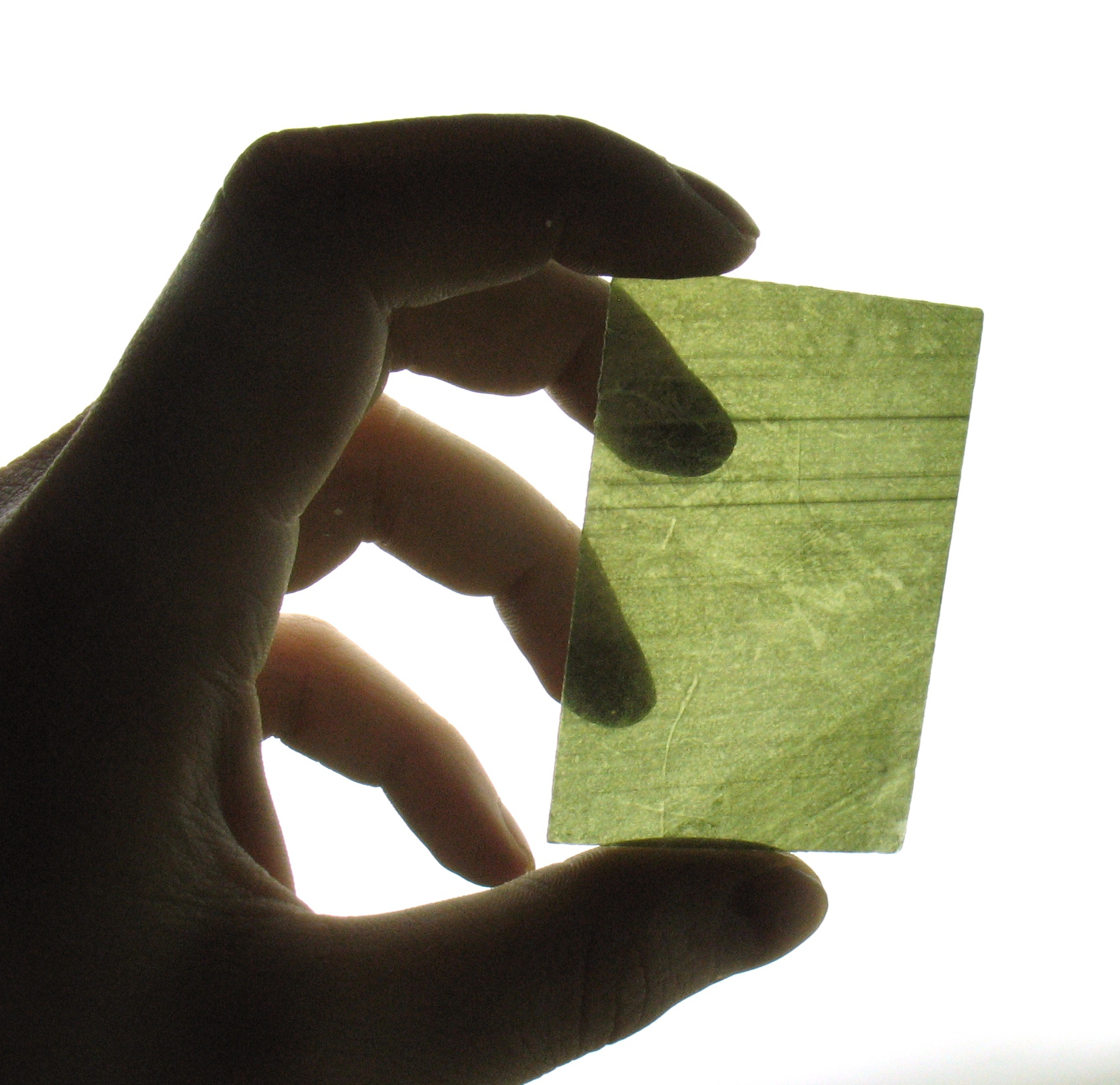
Natural mica is transparent. Mica obtained by processing natural mica is usually opaque.

A window with mica inserts from the exposition of the Krasnoyarsk Museum of Local History
Mica is a fairly soft material, mica plate (like most materials based on it) is easily cut with scissors. Due to its layered nature, gluing of mica is an unreliable occupation, the adhesion force between the layers is low, therefore, in the production of parts from mica, they fasten mechanically with rivets, eyelets, screws, etc.
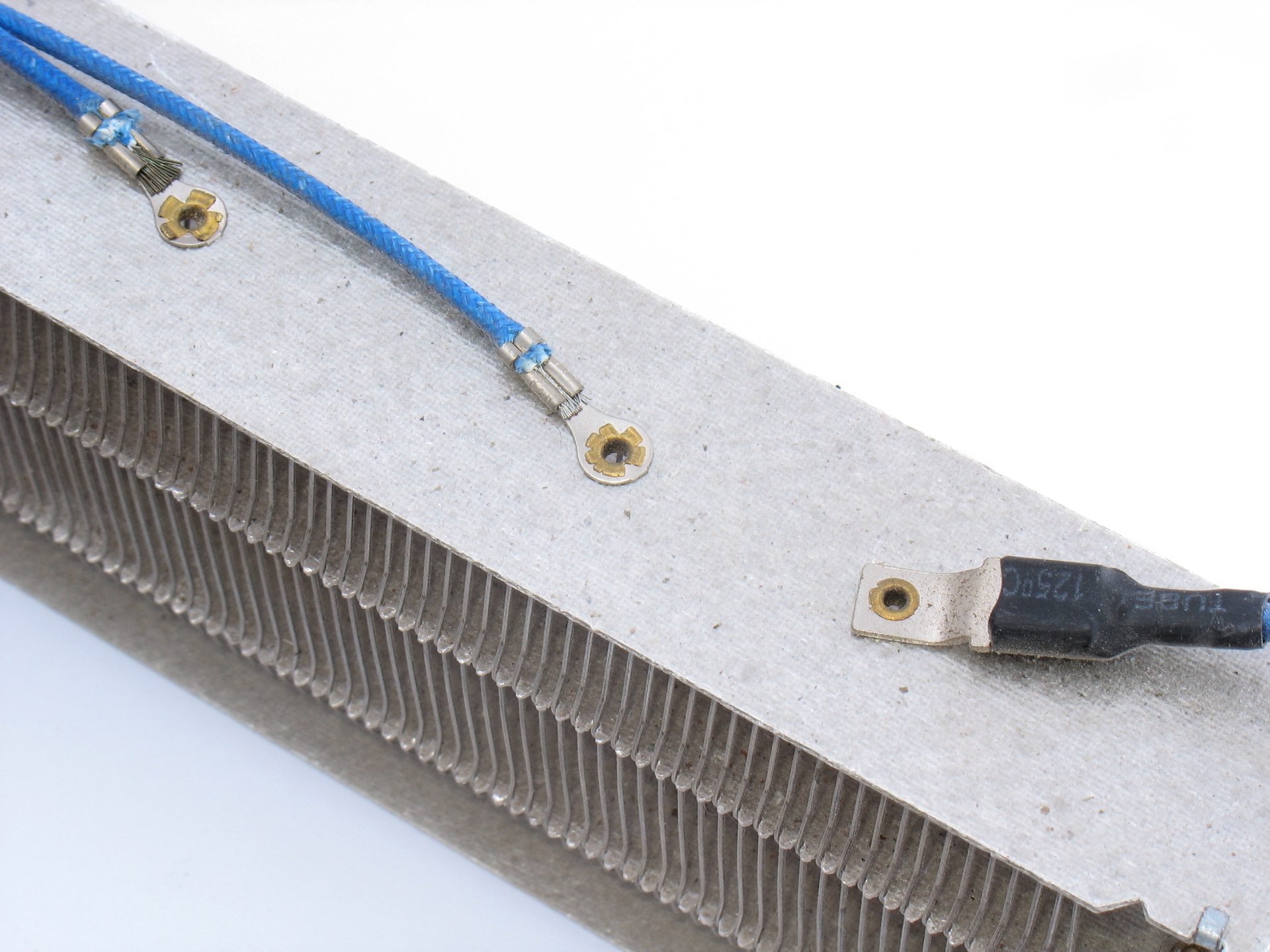
Electrical connections to the heating element are made with hollow rivets.
Very similar in appearance to porcelain, only better. Contain almost pure Al 2 O 3 . More well described in this article.
Solid, durable ceramics, which is made of:
Chip cases, usually in responsible use.

The processor cases were previously made of ceramic, but the growth of heat generation and price competition forced us to abandon this material. The anecdote about the new Russian and the tile in the bathroom from Intel was connected with the ceramic case of processors.
Electrovacuum equipment cases.

The case of the vacuum flask of the magnetron is made of copper and alumina ceramics. The pottery is visible in the photo, a purple belt between the cap and the case.
Aluminum oxide ceramics is very hard, treated like many ceramics with diamond tools. A piece of the ceramic body of the microcircuit is an excellent tool for writing messages on the windshield of a car, leaving clear even scratches no worse than a glass cutter.
This type of ceramics is dense, does not absorb moisture, keeps the vacuum, does not crack with a sharp temperature drop and thermal shock. At the same time, the adhesion of metal films to the surface is high, it allows making tracks on ceramics, and welding metal parts hermetically.
Unique, unrivaled material. Natural fiber, "mountain flax." It is a fireproof dielectric. Used in many applications, ranging from reinforcing additives in polymers, finishing insulation of heating devices. Available in the form of sheets, yarn, yarn. Most often it is used as a heat insulator, as a dielectric only in installations of low (up to 1 kV) voltage.

A piece of asbestos board and an old dirty asbestos cord. Asbestos is very soft to the touch and does not prick like fiberglass.
Widely used in construction. Slate is an asbestos fiber reinforced cement, a practically eternal material. Its low cost and fire resistance were highly valued. But there is one thing:
Asbestos is a carcinogen. Moreover, the carcinogenic class 1 (from IARC), along with arsenic, formaldehyde. Long-term observation has shown that asbestos products are dusted with fiber, which can cause lung disease, asbestosis, when inhaled. First of all, in the risk group, employees of enterprises for the extraction and processing of asbestos. Those who exploit asbestos products every day are less vulnerable. In other cases, there is no reason to panic, if you have a roof covered with slate at your dacha, and the stove in the bathhouse is covered with asbestos-cardboard, you will most likely die not from asbestos, but from diseases of the cardiovascular system ( mortality statistics ).
Asbestos and asbestos products are still widely produced, since in some tasks there is simply nothing (or too expensive) to replace asbestos without losing properties. Asbestos is an excellent material when designing experimental devices containing heaters or hot parts. On a piece of asbestos cardboard, you can calmly heat parts up to 1000 ° C with a gas burner, while it will retain its shape. Asbestos thread is convenient for tightening nichrome in heaters.
Bike (from Wikipedia):
This is absolutely counterintuitive, but this item is included here to blow your brain. Water practically does not conduct current! (UPD: while the publication was being prepared, an article about it appeared.) Everywhere they teach that water is a good conductor of electricity, and usually it is. But very pure deionized water, which contains nothing but H 2 O, does not conduct a current - its resistivity is 18 MΩcm. The water that conducts is not clean enough. Measuring electrical conductivity is a fairly simple way to assess the quality and purity of water.

A bottle of deionized water from a radio store. Printed circuit boards of electronic devices should be washed only with distilled or deionized water, otherwise the salts contained in the water can cause troubles.
Having strongly polar and mobile molecules, water is not only an insulator, but also has a very high dielectric constant — about 81 at room temperature (for most ordinary dielectrics it does not exceed 20-30). Capacitive moisture meters are based on this: a small amount of water between the plates of a capacitor dramatically increases its capacity.
Unfortunately, water is an excellent solvent, and substances dissolved in it usually form electrolytes. It is necessary to stand with distilled water in air, and it dissolves carbon dioxide in itself, forming an electrolyte - a weak solution of carbonic acid. Water is capable of dissolving the walls of the vessel in which it is located. The slightest admixture of salts, especially chlorides and sulfides of sodium, potassium, calcium, dramatically increases the conductivity of water. Therefore, in practice, water is no good as a dielectric.
Dielectrics can be gaseous. Dry air is a good dielectric, but in some problems its electrical insulating properties are insufficient. An example of a gaseous dielectric is sulfur hexafluoride, or “hexafluoride,” it is heavier than air and has breakdown voltage several times higher than that of air, which allows an electric machine to be made smaller.
A rather amusing experience, when breathing in helium, the voice of a person becomes higher with gas looking different - the voice becomes lower . Other videos : A pair of helium - sulfur hexafluoride. Since gas is heavier than air, a light boat can float in it.
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final

Welcome to CAT (TRAFFIC)
In addition to conductors, dielectrics are needed for the production of electronics. Depending on the conditions and tasks, different properties of the dielectric may be important: heat resistance, loss tangent, hygroscopicity, mechanical strength, etc.
The manual section with polymers is even more superficial. The fact is that the properties of the polymer material depend on the synthesis conditions, the additives introduced, the heat treatment, and the subsequent processing. Thus, two samples of polystyrene can vary significantly in properties. Manufacturers of plastics go to various tricks and manipulations with the composition, making important and not very changes. It's like with books, different editions of the same work, somewhere on a newsprint with a bad layout, and somewhere on a quality paper with color illustrations from a fashion artist. Both books are “Lord of the Rings”, but impressions of use may differ. Therefore, some general properties of different types of polymers are given; for more precise characteristics, refer to the reference book.
Materials that are used in electronic technology change as progress progresses. So, earlier it was widely used, for example, wood, silk, ebonite. Today, many materials have been superseded by cheaper, more technologically advanced substitutes. The manual has a description including historical materials, data for general development. Also added the information necessary for the completeness of the disclosure of the topic.
Inorganic dielectrics
Porcelain
Porcelain is dense solid ceramics obtained by burning a mixture of kaolin, quartz, feldspar and clay. It is similar to a porcelain cup in your kitchen, only less often it is coated with icing.
Application examples
High temperature insulators. In the form of porcelain beads to isolate the ends of the heating coils. The scaly-like construction allows for flexing without exposing the conductor.

The case of a mercury arc lamp from a light beam oscilloscope. Aluminum alloy frame, black body - carbolite, porcelain beads isolate the conductors that connect the lamp. The lamp is very hot during operation. A bunch of porcelain beads from various heaters.

Spark plugs from an internal combustion engine. The central electrode is insulated by porcelain. No other dielectric is able to withstand prolonged exposure to temperature, pressure, fuel inside the combustion chamber.
Details of electrical products. If you look inside the lamp holder, the part that contains the connecting lamellae is most likely made of porcelain, it can work for a long time at elevated temperature of the incandescent lamp without losing properties. Cases of fuses, sockets, holders of contacts of lamps - wherever there is a danger of heating, china is out of competition.

Holders of lamellae sockets, cartridge made of porcelain. The black body of ammunition - carbolite.

Power resistors have a porcelain tube base. The green resistor winding is hidden under the enamel.
Insulators on poles. In the photo there is an isolator from a pole that was liquidated during the reconstruction of the line. 30 years of the sun, wind, bird droppings, rain, frosts did not affect the porcelain, it still looks like new, it was enough to wash the insulator with soap.

Porcelain insulators of power lines. Between the porcelain insulator and the steel hook there is a polyethylene bush, to protect the porcelain from cracks. The disk form of the insulators allows water to flow down without forming a continuous layer that closes the conductor onto the support.
disadvantages
Fragile, like all ceramics. Tightened screw, blow - and porcelain showered.
Glass
Depending on the requirements, different types of glass can be used, from low-melting sodium to refractory quartz. The main plus of glass, in addition to its heat resistance, is transparency for visible light (and quartz glass is also transparent for ultraviolet). Also an important plus is the ability to visually assess the integrity, cracks are usually visible.
Application examples
Enclosures of radio tubes, lighting lamps, fuses. Quartz tubes - cases of heaters, electric grills.

Glass and porcelain insulator power lines working on the street for over 30 years.
Disadvantages.
Fragile, does not take blows. Some types of glass crack with sudden uneven heating.
A typical feature (but not obligatory!) Of quartz glass is a large number of cores in the direction of glass extrusion.
Interesting facts about glass
Here it is worthwhile to additionally say about sapphire glass, tempered glass and chemically tempered glass. In the advertising descriptions of many electronic devices for mass consumption can be found references to these types of glasses.
- A sapphire glass is not formally a glass (it is not amorphous, like glass, but crystalline), but due to external similarity, it is so called. Sapphire glass is thin plates of leucosapphire (pure Al 2 O 3 - alumina). Sapphire is harder than ordinary glass, therefore it is used to protect optics from dust, abrasive abrasion with grains of sand in military equipment, and in expensive household devices. Sapphire wristwatches will stay scratched longer. At the same time, obtaining sapphire glass of large size at a reasonable price is difficult, so we will see a tablet with sapphire glass soon.
- Strained glass. The glass resists compression well and poorly tensile. To increase the mechanical strength of the glass, it can be hardened - the glass is heated to high temperatures and rapidly and evenly cooled. As a result, mechanical stresses are formed in the glass, which increase the mechanical strength. Most often, glass hardening is done for safety. Ordinary glass, if you throw a stone at it, is broken into several rather large fragments that can cause serious injury. Tempered glass during the destruction gives a lot of small fragments, which are much safer. Therefore, all the glass in the car, in shopping centers, glass shelves of furniture - hardened. A tempered glass product is not subject to processing, if you try to cut a glass bathroom shelf, it will crumble into cotton, so hardening is performed after processing. Demonstration of the properties of tempered glass are Batavian tears.
- Chemically toughened glass. For example, often referred to as Gorilla glass. For thin plates of glass, the thermal quenching method is not suitable, therefore the plates of glass are treated in solution, which, for example, replaces sodium ion with potassium ion. Since the potassium ion is larger, the surface layers of glass “burst” as it were with larger atoms in the lattice, creating just the required mechanical stresses. As a result - this glass is stronger, it is better to resist scratches.
Mica
Mica. Natural layered material, has heat resistance, durability, excellent dielectric. Mica is a large class of layered minerals, of which muscovite is used mainly in technology and sometimes biotite and phlogopite.
In English, mica is Mica, hence derived names of materials based on mica - micanites, mikalenta, micafolium, mikaleks, etc.
Mica mined in the mine is disassembled, sorted. Large pieces are manually split into plates - this is plucked mica - transparent homogeneous plates. Such mica has the highest quality and is used for critical applications - in vacuum technology, radiation input / output windows, etc. Unfortunately, large homogeneous pieces of mica without defects are a rarity, therefore mica plates of various shapes are glued together, so it turns out micanite . If a cloth (fiberglass, paper) is used as a substrate for sticking mica plates , micalenta, micafolium, and glass mikanit will be obtained . Minor mica wastes are ground, and in the form of water slurry, they are cast onto the net, just like paper. After removing water, the particles of mica stick together in a single canvas - it turns out mica paper (mica, mica) . The resulting fabric for strength can be impregnated with an organic binder. The flexibility of mica paper allows it to be wound as insulation. Also winding can be obtained rods, tubes. If mica is impregnated with molten glass, the resulting durable material is called miklex .
Ground into mica dust - a component of pigments, thanks to its “scales” gives a pearl effect. Biotite is mainly used in pigments.
Synthetic material - fluoroflogopite (synthetic mica) is mica (phlogopite) where -OH groups are replaced by fluorine. Fluoroflogopit is more durable and thermally stable, looks also like mica, also layered but absolutely transparent / white, and not yellowish, like natural mica. Alas, as long as I did not come across this material.
Application examples
Structural elements for holding heating elements in hair dryers, heaters, fan heaters, soldering irons, etc.

Domestic fan heaters. The construction on the left is less material-intensive, but much less reliable, especially under mechanical loads.
As a protective window of the microwave radiation from the magnetron in the microwave. (Usually, when food gets mica, it becomes charred, and as a conductor, it starts to sparkle violently, which causes the microwave owners to throw out the microwave with fear, although it is enough to cut the mica out of the sheet and replace the window.)

Window output microwave radiation from mica.
Due to the fact that thin plates of mica do not let in gases, but they let in energetic charged particles - mica windows are used in the construction of alpha and beta particles counters.
Used in the construction of radio tubes - keeps the electrodes in place.

The octagonal plate is made of mica.
Used as mica capacitor material. Mica acts as a dielectric, and electrodes act as conductive metal deposition on mica plates. This type of capacitor is becoming less and less, replaced by capacitors based on polymer films. Mica capacitors can operate at high temperatures.

Mica capacitors produced in the USSR half a century ago.

Mica plates in the condenser. Metallization on the plates forms the plates.
Before the advent and widespread use of thermally conductive insulating gaskets made of polymeric materials like Nomakon, mica plates were used to electrically insulate the components while maintaining thermal contact, for example, when several transistors with different voltages needed to be attached to one radiator.

Records of natural plucked mica.
Interesting mica facts
Earlier, several centuries ago, when they did not know how to make thin window glass, translucent structures made splitting natural mica. Since large pieces of mica without defects were rare, the windows took on a bizarre shape.

Natural mica is transparent. Mica obtained by processing natural mica is usually opaque.

A window with mica inserts from the exposition of the Krasnoyarsk Museum of Local History
Mica is a fairly soft material, mica plate (like most materials based on it) is easily cut with scissors. Due to its layered nature, gluing of mica is an unreliable occupation, the adhesion force between the layers is low, therefore, in the production of parts from mica, they fasten mechanically with rivets, eyelets, screws, etc.

Electrical connections to the heating element are made with hollow rivets.
Aluminum Oxide Ceramics
Very similar in appearance to porcelain, only better. Contain almost pure Al 2 O 3 . More well described in this article.
Solid, durable ceramics, which is made of:
Chip cases, usually in responsible use.

The processor cases were previously made of ceramic, but the growth of heat generation and price competition forced us to abandon this material. The anecdote about the new Russian and the tile in the bathroom from Intel was connected with the ceramic case of processors.
Electrovacuum equipment cases.

The case of the vacuum flask of the magnetron is made of copper and alumina ceramics. The pottery is visible in the photo, a purple belt between the cap and the case.
Aluminum oxide ceramics is very hard, treated like many ceramics with diamond tools. A piece of the ceramic body of the microcircuit is an excellent tool for writing messages on the windshield of a car, leaving clear even scratches no worse than a glass cutter.
This type of ceramics is dense, does not absorb moisture, keeps the vacuum, does not crack with a sharp temperature drop and thermal shock. At the same time, the adhesion of metal films to the surface is high, it allows making tracks on ceramics, and welding metal parts hermetically.
Asbestos
Unique, unrivaled material. Natural fiber, "mountain flax." It is a fireproof dielectric. Used in many applications, ranging from reinforcing additives in polymers, finishing insulation of heating devices. Available in the form of sheets, yarn, yarn. Most often it is used as a heat insulator, as a dielectric only in installations of low (up to 1 kV) voltage.

A piece of asbestos board and an old dirty asbestos cord. Asbestos is very soft to the touch and does not prick like fiberglass.
Widely used in construction. Slate is an asbestos fiber reinforced cement, a practically eternal material. Its low cost and fire resistance were highly valued. But there is one thing:
Asbestos is a carcinogen. Moreover, the carcinogenic class 1 (from IARC), along with arsenic, formaldehyde. Long-term observation has shown that asbestos products are dusted with fiber, which can cause lung disease, asbestosis, when inhaled. First of all, in the risk group, employees of enterprises for the extraction and processing of asbestos. Those who exploit asbestos products every day are less vulnerable. In other cases, there is no reason to panic, if you have a roof covered with slate at your dacha, and the stove in the bathhouse is covered with asbestos-cardboard, you will most likely die not from asbestos, but from diseases of the cardiovascular system ( mortality statistics ).
Asbestos and asbestos products are still widely produced, since in some tasks there is simply nothing (or too expensive) to replace asbestos without losing properties. Asbestos is an excellent material when designing experimental devices containing heaters or hot parts. On a piece of asbestos cardboard, you can calmly heat parts up to 1000 ° C with a gas burner, while it will retain its shape. Asbestos thread is convenient for tightening nichrome in heaters.
Bike (from Wikipedia):
For a long time there is a legend about how Akinfiy Demidov brought to Peter I a beautiful snow-white tablecloth from his Ural plant. During the meal, he pointedly knocked over a plate of soup on the tablecloth, poured a glass of red wine, then crumpled up the tablecloth and threw it into the fireplace. Then, taking it out of the fire, he showed the king: there was not a single spot on it. This tablecloth was made of Ural chrysotile asbestos. In fact, Demidov serf workers achieved perfection in the manufacture of asbestos fabrics.
They made them openwork ladies' hats, gloves, wallets, handbags and lace. They did not require washing, they were thrown into the fire, and a few minutes after cooling, they could be worn again. With its elasticity asbestos fabric stronger than steel wire to break.
Water
This is absolutely counterintuitive, but this item is included here to blow your brain. Water practically does not conduct current! (UPD: while the publication was being prepared, an article about it appeared.) Everywhere they teach that water is a good conductor of electricity, and usually it is. But very pure deionized water, which contains nothing but H 2 O, does not conduct a current - its resistivity is 18 MΩcm. The water that conducts is not clean enough. Measuring electrical conductivity is a fairly simple way to assess the quality and purity of water.

A bottle of deionized water from a radio store. Printed circuit boards of electronic devices should be washed only with distilled or deionized water, otherwise the salts contained in the water can cause troubles.
Having strongly polar and mobile molecules, water is not only an insulator, but also has a very high dielectric constant — about 81 at room temperature (for most ordinary dielectrics it does not exceed 20-30). Capacitive moisture meters are based on this: a small amount of water between the plates of a capacitor dramatically increases its capacity.
Unfortunately, water is an excellent solvent, and substances dissolved in it usually form electrolytes. It is necessary to stand with distilled water in air, and it dissolves carbon dioxide in itself, forming an electrolyte - a weak solution of carbonic acid. Water is capable of dissolving the walls of the vessel in which it is located. The slightest admixture of salts, especially chlorides and sulfides of sodium, potassium, calcium, dramatically increases the conductivity of water. Therefore, in practice, water is no good as a dielectric.
SF6 gas
Dielectrics can be gaseous. Dry air is a good dielectric, but in some problems its electrical insulating properties are insufficient. An example of a gaseous dielectric is sulfur hexafluoride, or “hexafluoride,” it is heavier than air and has breakdown voltage several times higher than that of air, which allows an electric machine to be made smaller.
A rather amusing experience, when breathing in helium, the voice of a person becomes higher with gas looking different - the voice becomes lower . Other videos : A pair of helium - sulfur hexafluoride. Since gas is heavier than air, a light boat can float in it.
Links to parts of the manual:
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final
All Articles