Guide to electrical materials for all. Part 3
Continuing guidance on electrical materials. In this part we finish dismantling the conductors: Carbon, Nichromes, thermostable alloys, solders - tin, transparent conductors.

Welcome to CAT (TRAFFIC)
I want to say thank you to everyone for the efficient comments to the previous parts, my TODO list is growing. If the trend continues, I will not publish the final version of the manual in pdf format in part 11, as I planned, but in part 12th, together with a list of improvements and improvements. Leave suggestions in the comments which places require a more detailed explanation.
This part is devoted to "so-so conductors" - materials that conduct current, but do it very badly, and they accept it only because of some special properties of the material, which other conductors do not have.
C is carbon. Not really metal, but also a conductor. Graphite, coal dust are not such good conductors as metals, but they are very cheap and do not corrode.
Component resistors. In the form of films, in the form of bulk bars in a dielectric shell.
Additive in polymers to impart electrical conductivity. To protect against the formation of static electricity, it is enough to introduce finely dispersed graphite into the polymer, and the plastic from the dielectric becomes a very bad conductor, sufficient to allow a static charge to flow from it. When working with products made from such plastic, they will not stick and spark, which is important in case of fire hazard or working with electronics.

Conductive varnish based on graphite slurry.
Based on polymers filled with fine graphite, various heaters are based - film electric heaters of underfloor heating, heating cables for water supply systems, heaters for clothes, etc. The high coefficient of expansion of polymers during heating leads to negative feedback, which makes such heaters self-regulating and therefore safe. When current is passed through such a polymer, it heats up, expands from heating, the contact between the carbon particles in the polymer matrix deteriorates, this increases the resistance, the current flow decreases, and the heating decreases. As a result, some polymer temperature is established, which is stably maintained by this feedback mechanism without any external devices.

Heater from the stove laser printer. The base is porcelain, the conductors are silver. Heater - carbon composition, covered to protect the glaze.
Similarly arranged polymer self-resetting fuses. If the current through such a fuse exceeds the rated value, the polymer in the composition expands from heating, and a sharply increased resistance interrupts the current through the fuse to some small value. These fuses provide slow protection, but do not require replacing the fuse after every accident.
Coal welding electrode - used for welding, when the electrode is only required to maintain the arc without melting. Coal is much cheaper than tungsten, but less durable and gradually burns in air.

Electrodes from the arc lamp used for filming. The brand of KSB electrodes is Coal KinoFilming White Flame non-copper.
Copper-graphite materials. It is obtained by sintering copper and graphite powder in different proportions. Depending on the composition can be from black as coal to dark red with a copper sheen. Used as a material of sliding contacts - brushes of electrical appliances. Such brushes provide low resistance to rotation - they slide well along the collector contacts. In addition, their hardness is noticeably lower than the hardness of the collector metal, so that in the process of operation, cheap brushes, and not an expensive rotor, wear out and need to be replaced.

Worn brushes from the engine of the washing machine. Poor brush contact with the collector is the cause of increased arcing.
If an urgently needed carbon electrode is needed, for example, to make a thermocouple, the most affordable way is to pull the central electrode out of the salt battery (the label of which starts with R and not LR, alkaline (“alkaline”)) will not work). The carbon rod from the battery contains traces of electrolyte, so before use it will not be superfluous to rinse and boil it in water to remove residual electrolyte.
For the manufacture of heaters, powerful resistance alloys are required with the following requirements:
Heaters are usually made from the following alloys:
Nichrome (55-78% nickel, 15-23% chromium) operating temperature up to 1100 ° C although nichrome is a whole class of alloys with a small difference in composition.
Fekhral , the name is derived from the composition FeCrAl (12-27% Cr, 3.5-5.5% Al, 1% Si, 0.7% Mn, the rest Fe) working temperature up to 1350 ° C (sometimes called cantal - kanthal, it is not alloy grade, but a trade mark that has become common, such as "thermos").
The addition of chromium ensures the formation of a protective film on the alloy surface, thanks to which nichrome heaters can operate for a long time in air with a high surface temperature.
Fechral after heating becomes brittle. Nichrome after heating can still somehow bend. At the same time, fechral is cheaper than nichrome, in retail it is not so noticeable, but it is noticeable in wholesale lots.
The nichrome spiral with a wick inside - an evaporator of an electronic cigarette. Nichrome string heated by electric current, cut polystyrene foam. Thermal insulation sealers are also made of nichrome - currently the most reliable way to remove insulation from a wire and not damage the conductive core.
Surprisingly, it is quite difficult to buy nichrome in the form of wire in small quantities, local sellers do not even want to hear about quantities less than a kilogram. So, if you need to make a heating element, it is easier to rewind nichrome with some kind of faulty heater.
The ends of the heating elements are usually welded to tokovodam or clamped mechanically - with a screw or crimp.
All materials have TKS - temperature coefficient of resistance, a measure of how much the resistance varies with temperature. It can be positive - as with metals, with increasing temperature, the resistance increases, it can be negative, like with semiconductors, with increasing temperature, resistance decreases. In the manufacture of precision measuring devices, it is necessary to have resistance with a minimum nominal drift depending on temperature. To do this, invented alloys with a minimum TKS:
Constantan (59% Cu, 39-41% Ni, 1-2% Mn)
Manganin (85% Cu, 11.5-13.5% Mn, 2.5-3.5% Ni)
A table indicating the temperature coefficient (denoted as α) for various
metals:
If simplified, the coefficient α says how many times the resistance of the conductor will change when the temperature changes by one degree Celsius.
Soldering is the process of joining two parts using solder, a material with a melting point lower than that of the parts being joined. For example, the connection of two copper conductors using tin. It is the use of solder that is the main difference from welding, when parts are connected by melt from themselves, for example, a steel hook is welded to a steel door using a steel melting welding electrode.
Solders are more often classified into two groups - refractory (melting point 400 ° C and more) and low-melting. Or, sometimes, hard and soft. Given that soft solders are usually low-melting, often hard solders are synonymous with refractory, and soft solders are low-melting.
In electronic technology solders are used to create a reliable electrical contact. The main solders in electronic equipment are soft, based on tin and tin-lead alloys. All other exotic solders will not be considered.
Sn - Tin. The main component of soft solders. Tin is a relatively low-melting metal, which makes it possible to use it to connect conductors. In pure form is not used (see the facts). Due to the high cost of tin (as well as other reasons, see below), it is diluted in solders with lead. Solder from 61% of tin and 39% of lead forms a eutectic , such a mixture, POS-61 (Solder Tin-Lead - 61% of tin) solder radio components on the boards, wires. In less critical sites (chassis, heat sinks, screens, etc.), tin in solders is diluted more strongly, up to 30% tin, 70% lead.
Electronic devices were soldered for a long time by tin-lead solders. Then environmentalists came running and said that lead is a heavy metal, toxic, and there would be no problem if all these your iPhones, computers and other gadgets did not end up in a landfill from which lead enters the environment. Therefore, they invented a series of lead-free solders, when tin is diluted with bismuth, or completely used in pure form, stabilized with additives, for example, silver. But these solders are more expensive, worse in performance, more refractory. Therefore, tin-lead solders will remain for a long time in important products of military, space, medical use.
In addition, lead-free solders tend to form "whiskers." Tin whiskers - long thin crystals, growing from tin solder - the cause of failures and equipment failures. Unfortunately, additives in solders do not allow 100% stop the growth of "whiskers", so tin-lead solders, as time-tested, are used in critical systems - space, medicine, military, atomic applications. More about mustache.
On the basis of alloys containing tin, low-melting solders were developed. And even very fusible solders, which melt in hot water. A good list of alloys is in Wikipedia.

Coils and rods of tin-lead solders. The solder wire contains a central channel with a flux that facilitates the soldering process.
Basic solders for radio equipment
Completely fusible solders close the list:
They are used for the tinning of printed circuit boards by amateurs, because they melt in hot water, and you can quickly use copper rubber to seal the printed circuit board with a rubber trowel under a layer of boiling water. In the technique they are used for soldering parts that do not withstand heating to ordinary solder temperature, or in cases where for some reason a very low-melting metal is needed (for example, for a temperature sensor).
If you solder spring-loaded contacts with low-melting solder, you get a simple and reliable thermal fuse, when the temperature rises, the solder melts and the contacts break the circuit. True, the fuse will be disposable. In many Soviet TVs in the horizontal scanner unit there was protection from a conventional steel coil spring soldered to a low-melting solder. When overheating, including from a large current through the spring, it was sealed off and pulled off. These types of fuses are very good as fire protection.
For the manufacture of thermocouples using alloys resistant to high temperatures, but with a high Thermoelectric Voltage. More information about thermocouples can be found in the relevant literature.
Alloys:
By connecting two conductors of two different metals, thermocouples are obtained, for example, a type K thermocouple (THA - Hromel-Alumel Thermocouple). The most common pairs are: chromel-alumel, chromel-copel, copper-constantan (for low temperatures), platinum-platinum rhodium (for accurate measurements and for high temperatures).
Oxide India - Tin (Indium tin oxide or abbreviated ITO) is a semiconductor, but it has low resistance, and most importantly, a film of indium-tin oxide is transparent.
This property is used in the manufacture of LCD displays, the grid of electrodes on the surface of the glass is applied from indium tin oxide. Also resistive touch panels have a transparent conductive coating.
The ITO film is barely visible in the reflection, so that at least it was noticeably had to disassemble the LCD display:

Glasses from the LCD indicator of an electronic watch. The indicator was connected to the electronic circuit through the conductive gum, the contact strip is visible at the bottom of the glass.
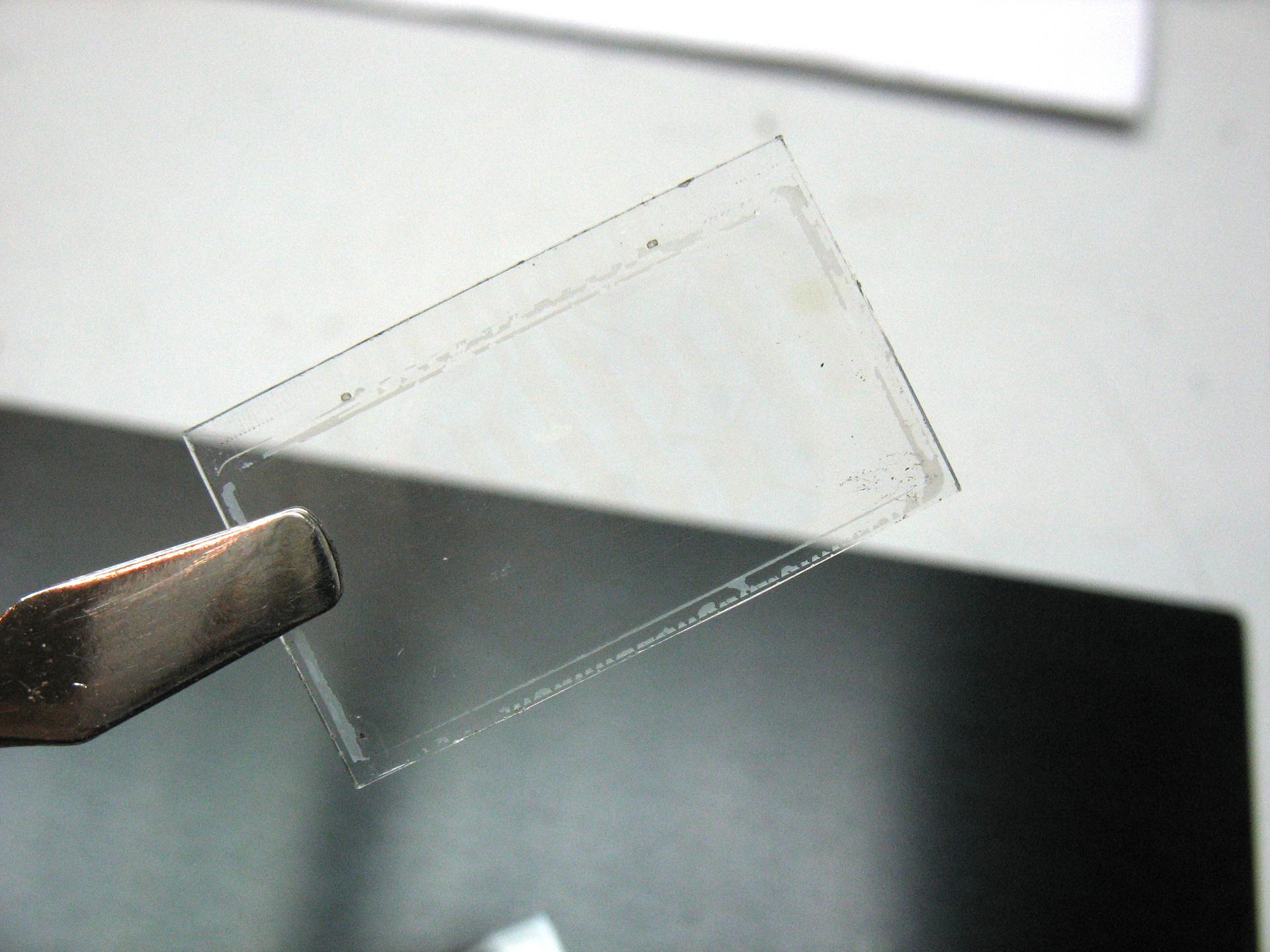
Conductive film not visible

Surprisingly, the resistance of the film is quite low.
At this point we finished the guides. In the next part we start the review of dielectrics.
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final

Welcome to CAT (TRAFFIC)
I want to say thank you to everyone for the efficient comments to the previous parts, my TODO list is growing. If the trend continues, I will not publish the final version of the manual in pdf format in part 11, as I planned, but in part 12th, together with a list of improvements and improvements. Leave suggestions in the comments which places require a more detailed explanation.
This part is devoted to "so-so conductors" - materials that conduct current, but do it very badly, and they accept it only because of some special properties of the material, which other conductors do not have.
Carbon
C is carbon. Not really metal, but also a conductor. Graphite, coal dust are not such good conductors as metals, but they are very cheap and do not corrode.
Application examples
Component resistors. In the form of films, in the form of bulk bars in a dielectric shell.
Additive in polymers to impart electrical conductivity. To protect against the formation of static electricity, it is enough to introduce finely dispersed graphite into the polymer, and the plastic from the dielectric becomes a very bad conductor, sufficient to allow a static charge to flow from it. When working with products made from such plastic, they will not stick and spark, which is important in case of fire hazard or working with electronics.

Conductive varnish based on graphite slurry.
Based on polymers filled with fine graphite, various heaters are based - film electric heaters of underfloor heating, heating cables for water supply systems, heaters for clothes, etc. The high coefficient of expansion of polymers during heating leads to negative feedback, which makes such heaters self-regulating and therefore safe. When current is passed through such a polymer, it heats up, expands from heating, the contact between the carbon particles in the polymer matrix deteriorates, this increases the resistance, the current flow decreases, and the heating decreases. As a result, some polymer temperature is established, which is stably maintained by this feedback mechanism without any external devices.

Heater from the stove laser printer. The base is porcelain, the conductors are silver. Heater - carbon composition, covered to protect the glaze.
Similarly arranged polymer self-resetting fuses. If the current through such a fuse exceeds the rated value, the polymer in the composition expands from heating, and a sharply increased resistance interrupts the current through the fuse to some small value. These fuses provide slow protection, but do not require replacing the fuse after every accident.
Coal welding electrode - used for welding, when the electrode is only required to maintain the arc without melting. Coal is much cheaper than tungsten, but less durable and gradually burns in air.

Electrodes from the arc lamp used for filming. The brand of KSB electrodes is Coal KinoFilming White Flame non-copper.
Copper-graphite materials. It is obtained by sintering copper and graphite powder in different proportions. Depending on the composition can be from black as coal to dark red with a copper sheen. Used as a material of sliding contacts - brushes of electrical appliances. Such brushes provide low resistance to rotation - they slide well along the collector contacts. In addition, their hardness is noticeably lower than the hardness of the collector metal, so that in the process of operation, cheap brushes, and not an expensive rotor, wear out and need to be replaced.

Worn brushes from the engine of the washing machine. Poor brush contact with the collector is the cause of increased arcing.
Sources
If an urgently needed carbon electrode is needed, for example, to make a thermocouple, the most affordable way is to pull the central electrode out of the salt battery (the label of which starts with R and not LR, alkaline (“alkaline”)) will not work). The carbon rod from the battery contains traces of electrolyte, so before use it will not be superfluous to rinse and boil it in water to remove residual electrolyte.
Nichromes
For the manufacture of heaters, powerful resistance alloys are required with the following requirements:
- Relatively high resistivity - otherwise the heater will have to be made long and thin, which will adversely affect durability.
- Resistance to air oxidation. If air enters the bulb of an incandescent lamp, the coil will burn very quickly. At high temperatures, the rates of chemical reactions increase, and the oxygen of the air begins to oxidize even metals that are resistant at room temperature.
- Have acceptable mechanical properties. Low plasticity and increased fragility will negatively affect the reliability of the product.
Heaters are usually made from the following alloys:
Nichrome (55-78% nickel, 15-23% chromium) operating temperature up to 1100 ° C although nichrome is a whole class of alloys with a small difference in composition.
Fekhral , the name is derived from the composition FeCrAl (12-27% Cr, 3.5-5.5% Al, 1% Si, 0.7% Mn, the rest Fe) working temperature up to 1350 ° C (sometimes called cantal - kanthal, it is not alloy grade, but a trade mark that has become common, such as "thermos").
The addition of chromium ensures the formation of a protective film on the alloy surface, thanks to which nichrome heaters can operate for a long time in air with a high surface temperature.
Fechral after heating becomes brittle. Nichrome after heating can still somehow bend. At the same time, fechral is cheaper than nichrome, in retail it is not so noticeable, but it is noticeable in wholesale lots.
The nichrome spiral with a wick inside - an evaporator of an electronic cigarette. Nichrome string heated by electric current, cut polystyrene foam. Thermal insulation sealers are also made of nichrome - currently the most reliable way to remove insulation from a wire and not damage the conductive core.
Surprisingly, it is quite difficult to buy nichrome in the form of wire in small quantities, local sellers do not even want to hear about quantities less than a kilogram. So, if you need to make a heating element, it is easier to rewind nichrome with some kind of faulty heater.
The ends of the heating elements are usually welded to tokovodam or clamped mechanically - with a screw or crimp.
Alloys for the manufacture of thermostable resistance
All materials have TKS - temperature coefficient of resistance, a measure of how much the resistance varies with temperature. It can be positive - as with metals, with increasing temperature, the resistance increases, it can be negative, like with semiconductors, with increasing temperature, resistance decreases. In the manufacture of precision measuring devices, it is necessary to have resistance with a minimum nominal drift depending on temperature. To do this, invented alloys with a minimum TKS:
Constantan (59% Cu, 39-41% Ni, 1-2% Mn)
Manganin (85% Cu, 11.5-13.5% Mn, 2.5-3.5% Ni)
A table indicating the temperature coefficient (denoted as α) for various
metals:
| Material | Temperature coefficient α |
|---|---|
| Silicon | -0.075 |
| Germanium | -0.048 |
| Manganin | 0,00002 |
| Constantan | 0,00005 |
| Nichrome | 0.0004 |
| Mercury | 0.0009 |
| Steel 0.5% C | 0,003 |
| Zinc | 0,0037 |
| Titanium | 0,0038 |
| Silver | 0,0038 |
| Copper | 0,00386 |
| Lead | 0,0039 |
| Platinum | 0,003927 |
| Gold | 0,004 |
| Aluminum | 0,00429 |
| Tin | 0,0045 |
| Tungsten | 0,0045 |
| Nickel | 0,006 |
| Iron | 0,00651 |
If simplified, the coefficient α says how many times the resistance of the conductor will change when the temperature changes by one degree Celsius.
Solders
Soldering is the process of joining two parts using solder, a material with a melting point lower than that of the parts being joined. For example, the connection of two copper conductors using tin. It is the use of solder that is the main difference from welding, when parts are connected by melt from themselves, for example, a steel hook is welded to a steel door using a steel melting welding electrode.
Solders are more often classified into two groups - refractory (melting point 400 ° C and more) and low-melting. Or, sometimes, hard and soft. Given that soft solders are usually low-melting, often hard solders are synonymous with refractory, and soft solders are low-melting.
In electronic technology solders are used to create a reliable electrical contact. The main solders in electronic equipment are soft, based on tin and tin-lead alloys. All other exotic solders will not be considered.
Tin
Sn - Tin. The main component of soft solders. Tin is a relatively low-melting metal, which makes it possible to use it to connect conductors. In pure form is not used (see the facts). Due to the high cost of tin (as well as other reasons, see below), it is diluted in solders with lead. Solder from 61% of tin and 39% of lead forms a eutectic , such a mixture, POS-61 (Solder Tin-Lead - 61% of tin) solder radio components on the boards, wires. In less critical sites (chassis, heat sinks, screens, etc.), tin in solders is diluted more strongly, up to 30% tin, 70% lead.
Electronic devices were soldered for a long time by tin-lead solders. Then environmentalists came running and said that lead is a heavy metal, toxic, and there would be no problem if all these your iPhones, computers and other gadgets did not end up in a landfill from which lead enters the environment. Therefore, they invented a series of lead-free solders, when tin is diluted with bismuth, or completely used in pure form, stabilized with additives, for example, silver. But these solders are more expensive, worse in performance, more refractory. Therefore, tin-lead solders will remain for a long time in important products of military, space, medical use.
In addition, lead-free solders tend to form "whiskers." Tin whiskers - long thin crystals, growing from tin solder - the cause of failures and equipment failures. Unfortunately, additives in solders do not allow 100% stop the growth of "whiskers", so tin-lead solders, as time-tested, are used in critical systems - space, medicine, military, atomic applications. More about mustache.
Tin facts
- Pure tin is subject to “tin plague” when, at temperatures below 13.2 ° C, tin changes its crystal lattice, turning from a shiny metal to a gray powder (as when heated, a diamond turns into graphite). According to bikes, tin plague is one of the causes of the defeat of the Napoleonic army in the conditions of the harsh Russian cities (imagine how in the cold your buttons, spoons, forks, mugs turn into gray powder). And it was quite a fact that tin plague was one of the reasons that destroyed the Scott expedition - cans, containers with fuel were soldered with tin and in the cold just collapsed. A small addition of bismuth almost eliminates tin plague .
- Tin conducts electric current 7 times worse than copper.
- Tin is used as a protective coating for cans - tinned tin when in contact with food does not make it dangerous. (but since tin is to the right of iron in a series of metal tensions, tinning does not protect iron from corrosion galvanically, like zinc, which is to the left of iron in a series of tension. How galvanic protection works can be found here ).
- Prior to the wide distribution of aluminum, the foil was made of tin, it was called "Stanill" (from Stannum - the Latin nenanning of tin).
- Do not attempt to repair jewelry with soft tin and tin-lead solders. The strength of the connection will be unacceptable, and the presence of low-melting solder on the surface will complicate the normal soldering with hard solders.
Fusible solders
On the basis of alloys containing tin, low-melting solders were developed. And even very fusible solders, which melt in hot water. A good list of alloys is in Wikipedia.

Coils and rods of tin-lead solders. The solder wire contains a central channel with a flux that facilitates the soldering process.
Basic solders for radio equipment
- POS-61 - 61% tin, the rest is lead. Melting point (liquidus) 183 ° C. There are many similar in composition and properties of imported solders, in which the proportions of the components differ by a couple percent, for example, Sn60Pb40 or Sn63Pb37.
- POS-40 - 40% tin. The rest is lead. Melting point (liquidus) 238 ° C Less durable, more refractory, non-eutectic (does not melt immediately, there is a temperature range at which the solder is more like a porridge). But due to the fact that it is almost two times cheaper (tin is expensive), it is used for non-responsible connections - soldering of screens and tires. The solders POS-33 (melting point 247), POS-25 (melting point 260), POS-15 (melting point 280) are similar.
- Lead-free solders. For soldering copper plumbing pipes, the torch most often uses soft solder with 3% copper (Sn97Cu3). It does not contain lead, so it is suitable for drinking water. For environmental reasons, modern electronics in factories are mostly soldered with lead-free solders. Good article .
Completely fusible solders close the list:
- Rose Alloy: 25% Sn, 25% Pb, 50% Bi. Melting point +94 ° C.
- Wood alloy: 12.5% Sn, 25% Pb, 50% Bi, 12.5% Cd Melting point +68.5 ° C.
They are used for the tinning of printed circuit boards by amateurs, because they melt in hot water, and you can quickly use copper rubber to seal the printed circuit board with a rubber trowel under a layer of boiling water. In the technique they are used for soldering parts that do not withstand heating to ordinary solder temperature, or in cases where for some reason a very low-melting metal is needed (for example, for a temperature sensor).
If you solder spring-loaded contacts with low-melting solder, you get a simple and reliable thermal fuse, when the temperature rises, the solder melts and the contacts break the circuit. True, the fuse will be disposable. In many Soviet TVs in the horizontal scanner unit there was protection from a conventional steel coil spring soldered to a low-melting solder. When overheating, including from a large current through the spring, it was sealed off and pulled off. These types of fuses are very good as fire protection.
Other conductors
Thermocouple alloys
For the manufacture of thermocouples using alloys resistant to high temperatures, but with a high Thermoelectric Voltage. More information about thermocouples can be found in the relevant literature.
Alloys:
- Chromel (90% Ni, 10% Cr)
- Copel (43% Ni, 2-3% Fe, 53% Cu)
- Alumel (93-96% Ni, 1.8-2.5% Al, 1.8-2.2% Mn, 0.8-1.2% Si)
- Platinum (100% Pt)
- Platinum-rhodium (10-30% Rh)
- Copper (100% Cu)
- Constantan (59% Cu, 39-41% Ni, 1-2% Mn)
By connecting two conductors of two different metals, thermocouples are obtained, for example, a type K thermocouple (THA - Hromel-Alumel Thermocouple). The most common pairs are: chromel-alumel, chromel-copel, copper-constantan (for low temperatures), platinum-platinum rhodium (for accurate measurements and for high temperatures).
Oxide India-Tin
Oxide India - Tin (Indium tin oxide or abbreviated ITO) is a semiconductor, but it has low resistance, and most importantly, a film of indium-tin oxide is transparent.
This property is used in the manufacture of LCD displays, the grid of electrodes on the surface of the glass is applied from indium tin oxide. Also resistive touch panels have a transparent conductive coating.
The ITO film is barely visible in the reflection, so that at least it was noticeably had to disassemble the LCD display:

Glasses from the LCD indicator of an electronic watch. The indicator was connected to the electronic circuit through the conductive gum, the contact strip is visible at the bottom of the glass.

Conductive film not visible

Surprisingly, the resistance of the film is quite low.
At this point we finished the guides. In the next part we start the review of dielectrics.
Links to parts of the manual:
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final
All Articles