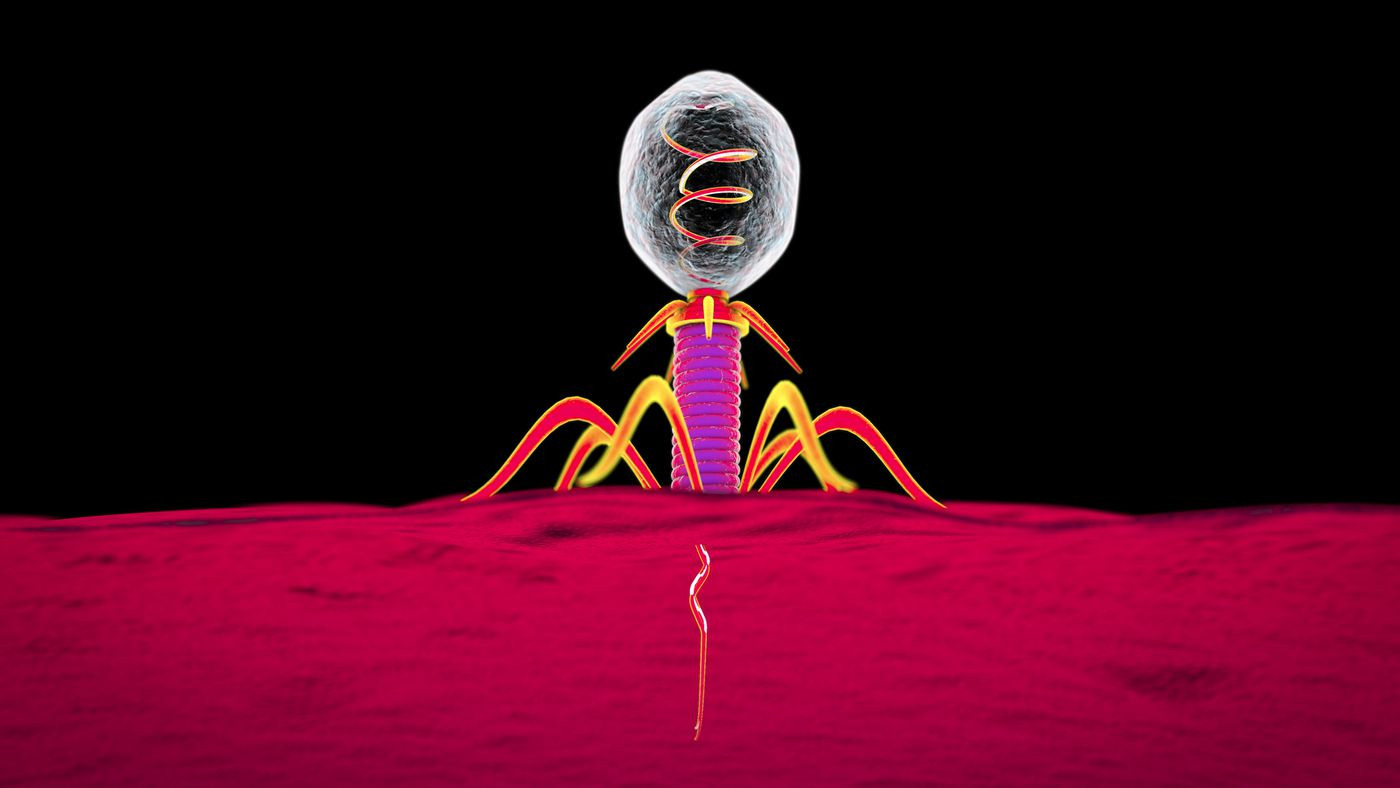
( c ) Phage therapy
91 years have passed since the discovery of penicillin, the first drug to revolutionize the effectiveness of treating bacterial diseases.
For almost a century of the existence of antibacterial drugs, many diseases have almost been forgotten. So, since 1947 it is believed that Yersinia pestis, the plague bacterium, the culprit of the death of 150 million people, was defeated. And the main means of the first stage of treatment were antibiotics.
However, due to the growing likelihood of the return of the forgotten and the emergence of new infectious diseases, the World Health Organization has been sounding the alarm over the past decade. The causes of the disaster were negligence, stupidity and evolution. Soon, even a normal cut on the finger can end in death. But new methods of struggle are coming.
First problems
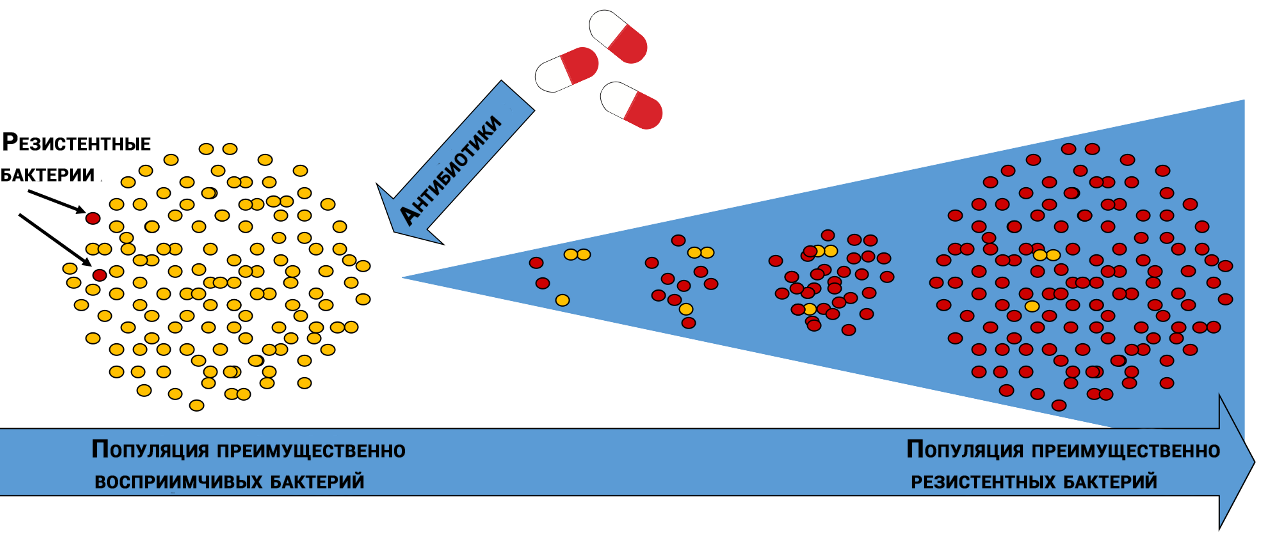
Natural selection of antibiotic-resistant bacteria
In different bacterial populations, molecules that are affected by antibiotics have different “sensitivities”. In addition, bacteria of the same species can differ from each other, like snowflakes - through diversity, life always finds its way.
By chance, some bacteria are less susceptible to the action of the antibiotic, and some of the bacteria that survived after the genocide will give offspring that will grow and die, but will have time to leave their offspring, which in a series of generations will create an “ideal” population.
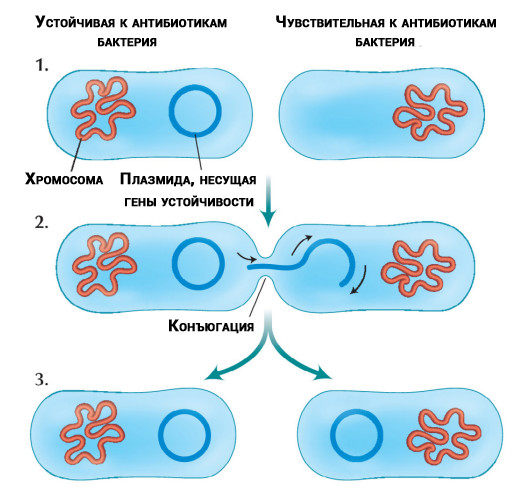
Transfer of antibiotic-resistant DNA molecules (plasmids) isolated from chromosomes between bacteria
Misfortune never comes alone. Researchers have discovered that some bacteria can transfer resistance genes to other bacteria of the same species or different. Transfer occurs regardless of whether antibiotics are present in the treatment or not.
Since the discovery of penicillin, the excessive use of antibiotics has significantly affected the development of bacterial resistance - immunity to antimicrobials, which led to the emergence of incurable super infections.
Cross the Rubicon
To reduce the spread of antimicrobial resistance, the AWaRe tool (Access, Watch and Reserve), developed by WHO experts in the form of a list of the main effective drugs, is currently used.
In this list, antibiotics are divided into three groups: Access (availability, first-choice drugs), Watch (vigilance) and Reserve (reserve). It determines which antibiotics should be used only for specific bacteria that cause the most common diseases, which significantly increase the risk of resistance, and which should be used in extreme cases, with infections with multiple resistance.
In practice, the algorithm operates as follows. Discharge from the patient’s body (pus, wound, scraping from the bronchus, etc.) is collected in a sterile vial and sent to a bacteriological laboratory, where it is determined which bacterium was in the material, to which antibiotics it was sensitive, and to which it was resistant .
If the bacterium is not sensitive to first-line antibiotics, doctors turn to less-used drugs. Some of them should always be in stock and not be used where other medicines can handle. Accordingly, microorganisms are less familiar with them and are still sensitive.
Ironically, among the antibiotics of the reserve, there are drugs derived from the bacteria themselves. For example, colistin, produced in 1949, is derived from the bacterium Paenibacillus polymyxa. This antibiotic, like others from the group of the last reserve, was used, as was believed, rarely. However, in 2015, colistin-resistant bacteria were found.
More than 100 countries have developed national plans to combat antimicrobial resistance, but 75% of them do not have the means or motivation to fund the plan for the proper use of antibiotics.
In particular, China, in spite of international recommendations, used 12 thousand tons of colistin (reserve preparation) per year for fattening pigs and preventing diseases in other animals.
In the absence of a clear understanding of why total control of the spread of resistance to antimicrobials is necessary, mathematics comes to the aid of researchers around the world, which allows you to simulate various situations and calculate the risks of developing resistance, including fatal ones. Science with heartless accuracy shows what threatens the world.
Pharmaceutical fiasco
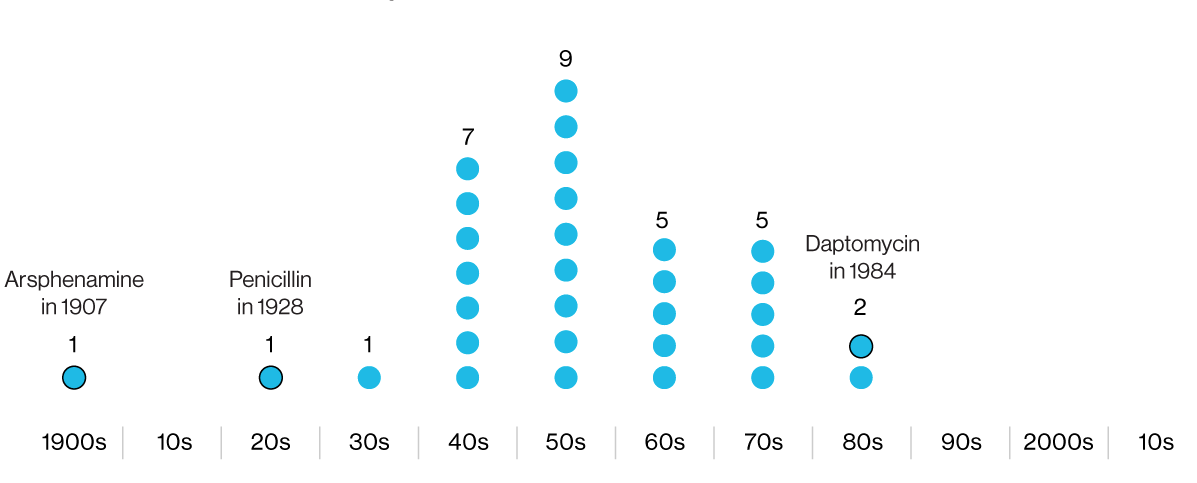
The infographics indicate the years of development of antibiotics, subsequently conducted in clinical practice. The first is arsphenamine, a dangerous arsenic-containing antimicrobial chemical. The latter, daptomycin, has lost effectiveness against some strains of MRSA.
After the penicillin revolution on the battlefields during World War II, the pharmaceutical industry entered the “golden era” of antibiotics. Companies hired researchers, missionaries, and travelers from around the world to collect soil samples in search of new antibacterial compounds. But the "golden age" rapidly came to naught, and in the 1980s came the "winter of antibiotics", which continues to this day.
Today, the development of fundamentally new antibiotics is a rare event. Of the fifty drugs in various stages of clinical trials, less than a dozen are truly innovative.
Investors are guided by logic, not emotions: the cost of such drugs is difficult to increase, since the public can be outraged, and you should not rely on regular deliveries - for the reason that the drug can be put on standby.
In addition, after spending years searching for a new substance and clinical trials of efficacy and safety, pharmaceutical companies run the risk of catching resistance just months after they enter the market.
Of all the randomized trials tested in double-blind, placebo-controlled trials in recent years, only eight antibiotics or combinations thereof have been approved: delafloxacin, meropenem + waborbactam, ozenoxacin, plasomycin, eravacycline, imipenem + cilastatin + relebactam, lefamulin.
Lefamulin, manufactured under the Xenlent brand, was the first drug approved in 20 years with a new mechanism of action against community-acquired bacterial pneumonia. It is significant that the manufacturer can get nothing from the unconditional medical triumph.
This has already happened . So, the company Achaogen, having spent $ 250 million, for 15 years tried to bring to the market a new antibiotic - plasomycin, after which it suddenly went bankrupt.
Given the relevance of the problem, other researchers are looking for new pragmatic approaches in developing methods to inhibit bacterial growth.
Riders of optimism

The startup has developed a platform for reprogramming bacteria on a genetic bioprinter that prints DNA fragments up to 10 thousand base pairs long.
Boston-based biotechnology company Ginkgo Bioworks, supported by the US military DARPA and venture capital fund Y Combinator, instead of more effective antibiotics, creates probiotics on the bioprinter - useful bacteria aimed at combating “competing” resistant microorganisms.
Locus Biosciences experts are developing an equally impressive product using the CRISPR Cas3 gene editing method. CRISPR technology is known for using the Cas9 enzyme, which acts as a “genetic scissor” for cutting, editing, and replacing DNA fragments.
Cas3 exceeds Cas9 in breadth - it is able to influence long sections of DNA. CRISPR Cas3 purposefully acts on bacteria and viruses, and not only cuts, but effectively erases certain DNA sequences - up to 100 thousand pairs of nucleotides at a time.
Locus Biosciences plans to use this technology to control dangerous bacteria. In favor of the method, the large size of the Cas3 enzyme plays - it acts on bacterial cells, but is too large to penetrate the human cell and damage our DNA.
I would like to finish the article filled with death and disappointment on a major note. We are not alone in the fight against bacteria. For billions of years, bacteriophage viruses have remained one of the most powerful tools for controlling microbial populations, but have hardly been studied due to the success of antibiotics.
The phages are busy doing what they love - they attack the bacterium .
After the Second World War, a paradoxical situation developed. In the USA and Western Europe, bacteriophages remained the focus of attention only of biologists, and in the countries of the Eastern bloc, real drugs were created from bacteriophages. As a result, Russia has become a world leader in the number of registered bacteriophage preparations.
In the United States, a renaissance of bacteriophages has occurred only in recent years. In May 2019, the results of the use of a mixture of three types of bacteriophages on a patient with a difficult to treat infection caused by one of the representatives of non-tuberculous mycobacteria Mycobacterium abscessus were published. The results are encouraging - the patient is recovering.
Studies of a number of other natural and genetically modified strains of bacteriophages have already begun, but drugs available to the mass consumer that are effective against certain bacteria will appear only after a few years.
Despite achievements, we are at the very beginning of a long and grueling fight against bacteria - the main battle is ahead. So far, most phages have not been described or are not available for genetic manipulation.
Other tools to solve the antibacterial problem are also far from perfect. Meanwhile, without increasing the pace of chemical and pharmaceutical research, we are faced with difficult decades of global threats.
In September 2019, the Global Committee on Preparedness Monitoring ( GPMB) presented the World at Risk report, which recognized the real likelihood of a pathogen that could kill 50-80 million people in a new pandemic.