Useless Benefits: Synthesis of UV-Absorbing Chemicals from Cashew Nuts

Summer is steadily moving towards its logical conclusion, but this does not mean that sunny days will end with the advent of September. The beaches will still suffer from an overabundance of people (the words of a real introvert), and people will continue to suffer from the almost inalienable attribute of a beach holiday or summer cottage drowning - sunburn. Fortunately, now there are a lot of creams and lotions that can prevent a person from turning into a baked tomato. The problem is that most of these protective equipment is made from inorganic substances, which are obtained in one way or another from natural resources, of which, as we know, there are not many left. To solve this problem, according to scientists from the University of Witwatersrand University and their colleagues from Germany, Tanzania and Malawi, cashew nut husks can. What is so special about cashews, how have scientists turned the husk into a sunscreen, how much more effective is this product and what is it better than currently available on the market? We learn about all this from the report of the research group. Go.
Study basis
What is the biggest minus of petroleum products? Yes, there is an environmental aspect, an economic aspect, and sometimes even a geopolitical one. But the simplest answer to this question is that oil, and therefore all its derivatives, sooner or later (according to the latest information, sooner rather than later) will end. This was known for a very, very long time, but mankind was not in a hurry to take any action to find alternatives, as if there was still a “bag” of oil hidden in its underground. Now the situation is beginning to change for the better, and all because of the activation of the principle associated with the cock pecking in a soft place.
One alternative to petroleum products may be biomass from waste that is not used anywhere else. Cashew husk refers to just such. Of course, to say that nuts or, say, corn will save the world (I will show you Kuz'kin’s mother!) Means a little exaggeration. However, nothing is lying or disappearing for an economic person, and one ski on the balcony can still serve.
That is why the authors of the work we are considering today have decided to convert the "useless" cashew husk into a substance that can protect human skin from ultraviolet radiation.
The sun is good, such as your favorite sweets. But if you eat 10 kilograms, they will want to go outside and breathe fresh air. Simply put, what is busy is not sensible, i.e. all is well, in moderation. Ultraviolet is a rather harmful thing, but only if it is completely surrendered, i.e. lying naked on the beach for 3 hours in a row. Of course, many people want a beautiful tan, but it is better to create it gradually, sunbathing in the sun a little bit, and then hide in the shade. In addition to umbrellas and other overhead constructions of a shadow-generating nature, the previously mentioned sunscreens, sprays, lotions, etc. are very popular.
They often contain organic compounds, but this does not mean that such a cream is 100% organic (as is often written on the packaging for marketing purposes). Among the inorganic compounds present in creams, there are titanium dioxide, zinc dioxide, etc. By the way, about 5 million tons of titanium dioxide are produced per year, and the volume of cashew production is about 4 million tons.
From the point of view of chemistry, a great interest in cashew nuts is due to the presence of a sufficient amount of easily extractable phenolic components in its husk (or rather in the cashew husk liquid - CNSL - Cashew Nut Shell Liquid ). In addition to this, there are substances with antimicrobial, anti-inflammatory, antitumor, antioxidant and insecticidal properties.
Chemists put the main emphasis on the phenolic components of CNSL: cashew acid, cardol and cardanol.
Let's go back to our sheep, and more specifically to UV (ultraviolet). The most effective organic compounds exhibit a high degree of absorption of UV radiation in the range from 315–400 nm ( UVA * ) to 280–315 nm ( UVB * ).
UVA * - type A ultraviolet radiation - long-wavelength.It is among phenols that the very important UV absorbing molecules are found that possess intramolecular O – H ··· O bonds. Among the most famous representatives of this group of molecules, researchers distinguish the following (get ready for an articulation test): salicylates, 2-hydroxybenzophenones, 2,2'-dihydroxybenzophenones, 3-hydroxyflavones and xanthones.
UVB * - type B ultraviolet radiation - medium wave.
Also, molecules with O – H ··· N intramolecular bonds: 2- (2-hydroxyphenyl) benzotriazoles, 2- (2-hydroxyphenyl) -1,3,5-triazines and 2- (5 aryl-) 1,3,4-oxadiazol-2-yl) phenols.
The above language-breaking compounds scatter UV radiation through non-radiative deactivation through tautomerization * due to the mechanism of intramolecular proton transfer in an excited state ( ESIPT ).
Tautomerization * is a property of a substance that allows it to be in one of the mutually transient chemical states.The above compounds, the names of which I will not repeat in order to avoid a fracture of the tongue, are used not only in skin creams. For example, 2-hydroxy-4-methoxybenzophenone (oxobenzone) is part of many sunscreens and added to plastics to limit its degradation due to UV radiation.
Researchers note that in addition to petrochemical origin, the main disadvantage of modern UV protection agents is their extremely negative effect on aquatic ecosystems, due to the very weak and slow biological decomposition of these substances.
It turns out, wherever you spit, everywhere there is at least one negative effect on one positive effect. It follows from this that it is necessary to create a safer (both for humans and ecology), organic and effective anti-UV agent, which the researchers did.
Research results
And now from philosophical reasoning, we turn to complex chemical transformations and beautiful molecular schemes that even Picasso would be inspired by.
Chemical transformation chain
Initially, cardanol obtained from CNSL and anacardic acid were used as starting materials, and a number of classes of potential UV absorbers were prepared in short synthetic sequences. The hydrogen bond of the phenolic hydroxyl group with the C = X (X = O or N) chromophore fragment was a central design feature to ensure effective ESIPT. As a starting point, benzophenones and xanthones with neighboring hydrogen-bonded hydroxyl substituents * were synthesized.
Deputy * is an atom or group of atoms which, as a result of a chemical reaction, replace a hydrogen atom (H) in organic compounds.Based on the cashew acid obtained from CNSL, a benzyl protected methyl ester 1 was prepared.
Halogen-lithium exchange on substituted bromobenzenes 2a-c, available from xylochemicals, followed by addition of ester 1 (Scheme No. 1 below) led to the formation of protected benzophenones 3a-c with moderate to poor yields.

Scheme No. 1: the molecular schemes of the synthesized compounds are marked with numbers from 1 to 25 in schemes No. 1-7 for convenience.
Hydrogenolytic O-debenzylation of 3a - c gives benzophenones 4a-c in excellent yields. Benzophenone 4c contained the desired two hydrogen-bound phenol. Exposure to 4a on AlCl 3 affords the second desired compound 5 , while hydrogen-bound 2,2'-dihydroxybenzophenones ( 6 ) were obtained by treating 4b with BCl 3 .
Another benzophenone having two hydrogen-bonded phenolic hydroxy groups was synthesized using cardanol 7 derived from CNSL as starting material. The esterification * of compound 7 with benzoyl chloride in a green solvent of 2-Me-THF gives ester 8 in excellent yields.
Etherification * is the reaction of the formation of esters due to the interaction of acids and alcohols.This was followed by a Fris microwave photo rearrangement of compound 8 in the presence of AlCl 3 to produce benzophenone 9 . The oxygenation of compound 9 catalyzed by ruthenium CH gave 2.2'-dihydroxybenzophenone 10 with a yield of 61% (Scheme 2).
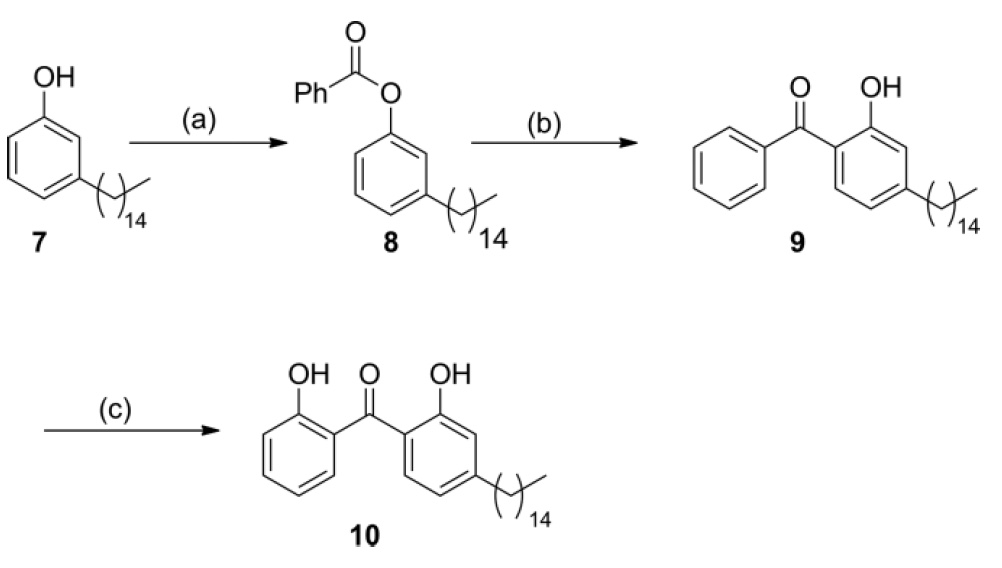
Scheme No. 2
Another class of potential ultraviolet absorbers having a hydroxyl group, where hydrogen is bonded to carbonyl, are 3-hydroxyflavones. Using cardanol 7 obtained from CNSL, the first step was carried out similarly to scheme No. 2 by initial acetylation of cardanol 7 to obtain compound 11 . Again, the Fris rearrangement using microwave radiation applied to compound 11 , when catalyzing AlCl 3 gave 1- (2-hydroxyphenyl) ethanone 12 in excellent overall yield.
Condensation of the aldol of compound 12 with benzaldehyde gave an intermediate compound of chalcone 13a , the oxidation of which with hydrogen peroxide made it possible to obtain the desired 3-hydroxyflavone 13b with a 54% yield in 2 stages of this process (Scheme No. 3).
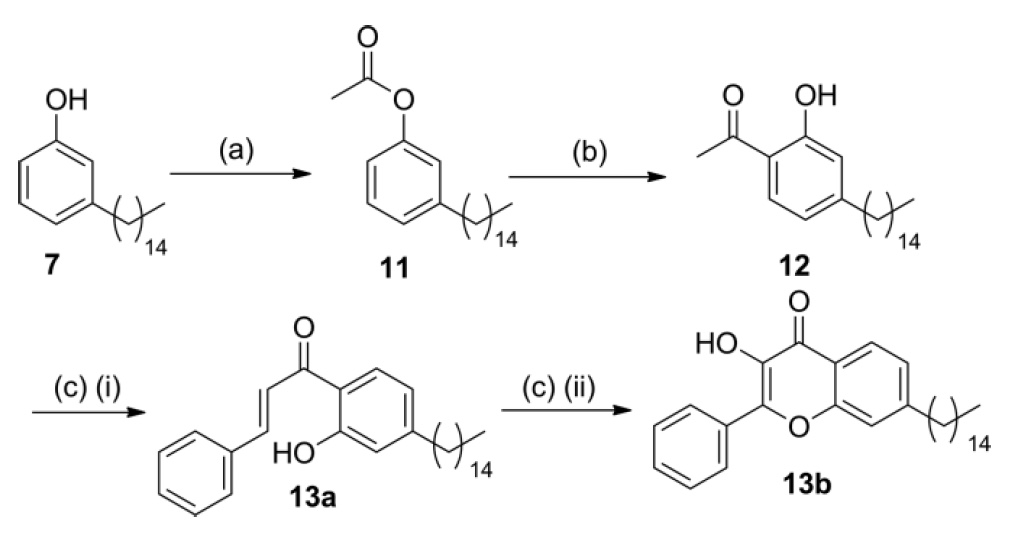
Scheme No. 3
Since acetic acid and benzaldehyde are xylochemicals, that is, they can be obtained from wood and related biomass species, we can safely say that the entire molecular skeleton 13a and 13b is obtained from renewable carbon.
The next class of compounds studied as potential absorbers of UV rays were xanthones bound by hydrogen. As an example, 1,8-dihydroxyxanton 14 was synthesized from cardanol.
Applying again Fris's rearrangement to compound 15, we managed to obtain benzophenone 16 (Scheme No. 4) with a yield of 84% in 2 runs.
The use of reflux * of compound 16 with potassium carbonate in acetone led to the formation of xanthon 17. The oxygenation of compound 16 catalyzed by ruthenium CH yielded 1.8-dihydroxyxanthone 14 from 54% yields in 2 runs.
Reflux * - partial condensation of mixtures of various vapors and gases for their enrichment with low boiling components.

Scheme №4
Scientists note, given that 2-fluorobenzoic acid can be obtained from natural anthranilic acid, the entire molecular skeleton in compound 14 is formally excreted by xylochemistry.
In addition to the above compounds, it is believed that nitrogen-containing substances (for example, 2-hydroxytriazines) can also perfectly cope with the absorption of UV radiation due to the intramolecular hydrogen bond OH ··· N. In this study, taking into account this fact, 2- (4,6-diphenyl-1,3,5-triazin-2-yl) phenol 20 .
SnCl 4- mediated formylation of cardanol 7 afforded benzaldehyde 18 with an excellent yield, followed by LiAlH 4 reduction of aldehyde 18 to give benzyl alcohol 19 (Scheme 5). The action of alcohol 19 on benzamidine under the catalysis of Cu (OAc) 2 gives 2- (4,6-diphenyl-1,3,5-triazin-2-yl) phenol 20 with a yield of 62%.

Scheme №5
Another important element of future husk sunscreen is benzamidine, which can be obtained from benzoic acid or benzaldehyde. Scientists synthesized 2,2 ', 2' '(1,3,5-triazine-2,4,6-triyl) triphenol 21 to check UV absorption in the case of an increase in the number of hydrogen-bound intramolecular N atoms. It was assumed that this result could be achieved by means of formyl cardanol 18 (Scheme No. 6).
The reaction of aldehyde 18 with hydroxylamine hydrochloride and FeCl 3 in DMF (dimethylformamide) under reflux gives nitrile 22 , which can be trimerized * by microwave irradiation to obtain completely symmetric s-triazine 21 with a yield of 73%.
Trimerization * - the formation of an oligomer (a molecule from a chain of identical units) of three molecules.
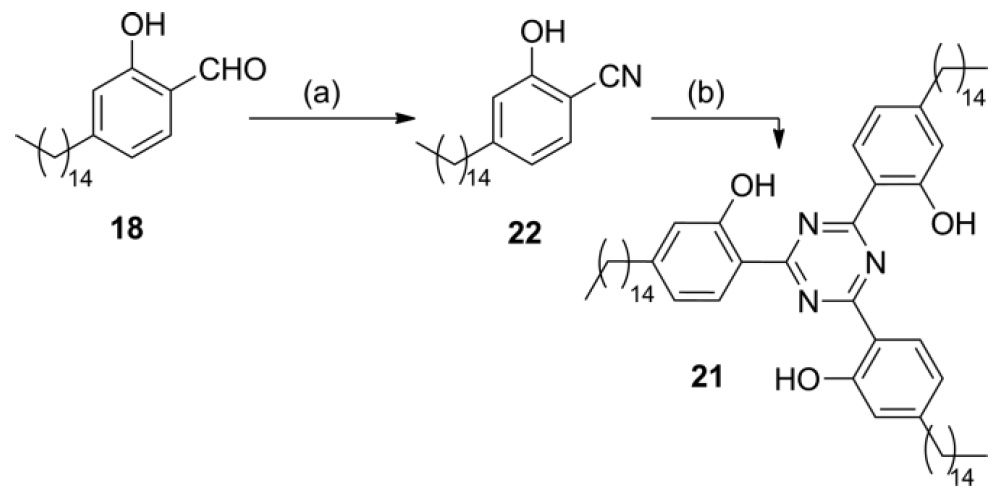
Scheme 6
The final compound (thank God), which should also show the hydrogen bond N ··· H and, therefore, in theory, be a good UV absorber, is oxadiazole 23 . Exposure of aldehyde 18 to veratric acid hydrazide ( 24 synthesized from veraldehyde) gives compound 25 . The oxidative formation of the oxadiazole ring gives 23 with a total yield of 39% in two runs (Scheme 7).
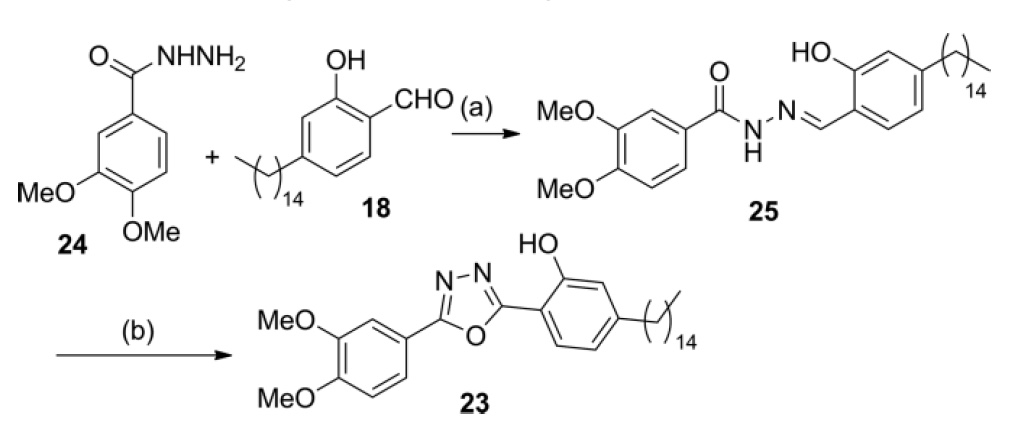
Scheme number 7
An important feature of compound 23 is that veraldehyde is obtained from xylochemicals, which means that the entire carbon skeleton of this compound can be obtained from organic matter and without any petrochemical elements.
So, we have gone through this long and exciting way of converting one into another and another into completely different (and alchemy is said to be fiction), now it’s worth getting acquainted with the properties of the compounds obtained, in particular with their ability to withstand ultraviolet radiation.
Analysis of UV profiles of synthesized compounds
The synthesized compounds 4c, 5, 6, 9, 10, 13a, 13b, 14, 20, 21, and 23 were analyzed for their UV absorption, as a result of which their molar extinction coefficients ( ε ) were obtained. All compounds were tested both with UVA radiation and with UVB, after which their results were compared with those that demonstrate commercially available protective agents (creams, spray, etc.).
The 2,2'-dihydroxybenzophenone ( 4c ) synthesized from anacardic acid showed quite good absorption of UV radiation in the UVA region with experimental values of ε 10538 L mol – 1 cm – 1 at 335 nm. In the case of 261 nm, which is not included in the UVA and UVB ranges, this compound showed a much better result: 36366 l⋅mol −1 ⋅cm −1 (image below).

Image No. 1: UV absorption profile of compound 4c , 5 and 6 , obtained from cashew acid.
For benzophenone 5, the values of ε were 8785 l mol – 1 cm – 1 at 374 nm and 18439 l mol – 1 cm – 1 at 265 nm.
Benzophenone 6 showed excellent UVA absorption with ε values 25014 L mol – 1 cm – 1 at 358 nm and 34096 mol – 1 cm – 1 at 287 nm.
For comparison, benzophenones 9 and 10 obtained from cardanol showed excellent absorption at the edge of the UVB region with ε values: 47909 at 279 nm and 31659 at 282 nm for mono- and dihydroxybenzophenones, respectively (image No. 2).
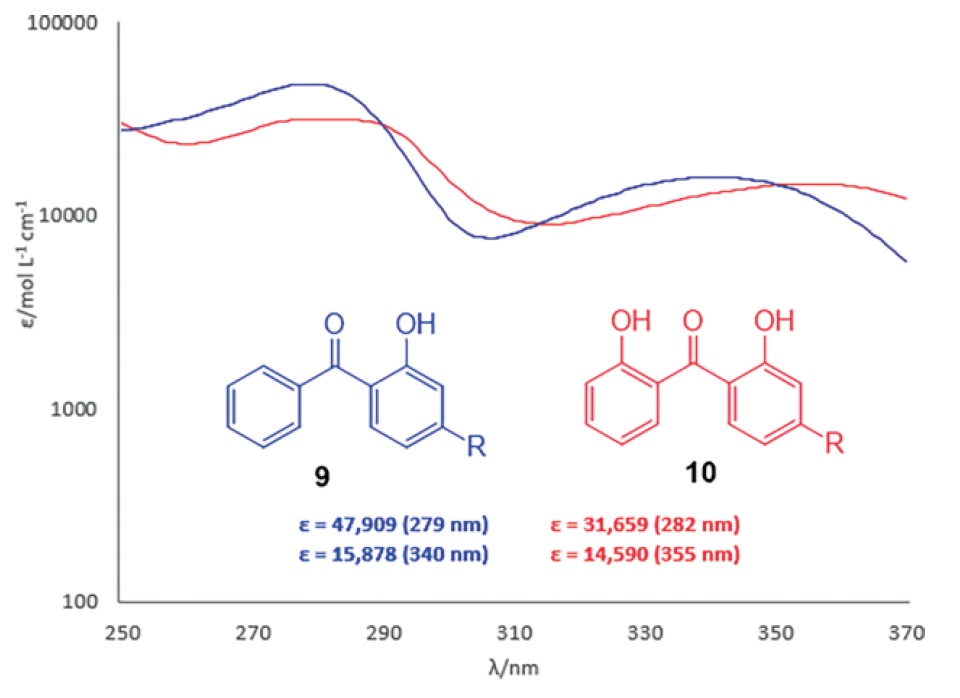
Image 2: UV absorption profile of benzophenones 9 and 10 obtained from cardanol.
3-hydroxyflavone 13b showed an excellent UVA absorption profile with ε 39870 at 314 nm and 44979 at 345 nm (image below).
Intermediate phenylpropenone 13a reached ε of 20403 at 314 nm and 10,250 at 347 nm. Dihydroxy xanton 14 also showed a good absorption profile, and its ε was 23765 L mol – 1 cm – 1 at 254 nm.

Image No. 3: Absorption profile of UV chalcon 13a , flavon 13b and xanton 14 .
S-triazine 21 showed better ultraviolet absorption in both the UVA and UVB regions with ε 21452 at 300 nm and 12515 at 364 nm. From these indicators it follows that it is compound 21 that is a UV blocking agent of a wide spectrum, since it has shown excellent performance in both regions of ultraviolet radiation.
But 2- (4,6-diphenyl-1,3,5-triazin-2-yl) phenol 20 showed excellent UV absorption at the beginning of the UVB region: ε = 29252 L mol – 1 cm – 1 at 278 nm.

Image No. 4: UV absorption profile of triazines 20 and 21 .
Oxadiazole 23 showed moderate ultraviolet absorption in the UVA region, and its ε was 6367 at 326 nm and 3373 at 293 nm (image No. 5).
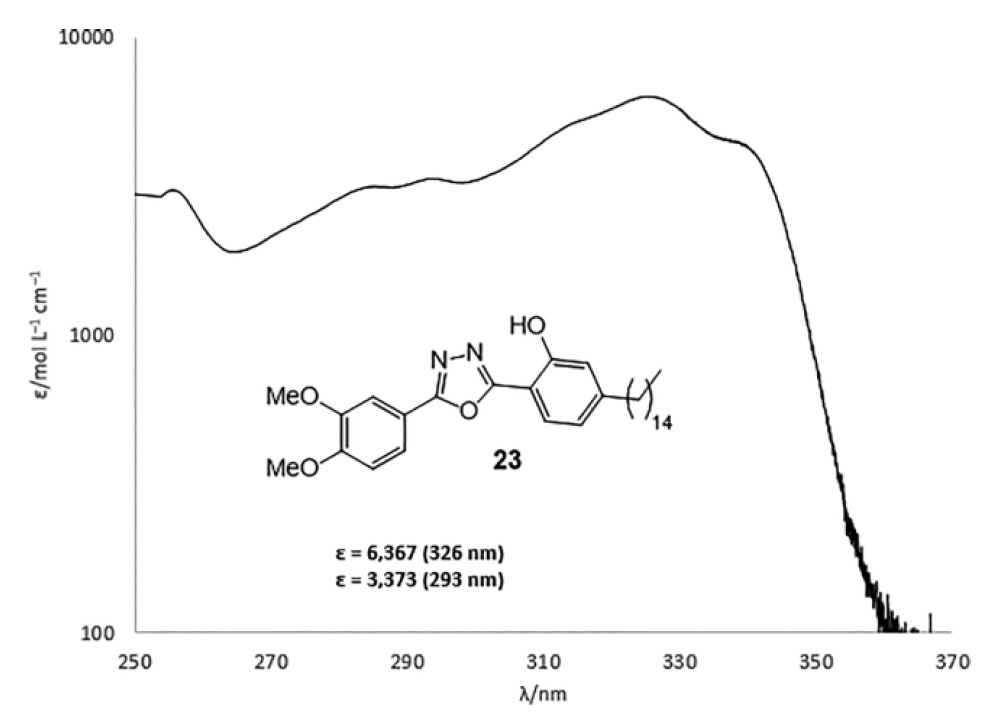
Image No. 5: UV absorption profile of oxadiazole 23 .
Regarding commercially available sunscreens, the most common ones had the following results:
- oxybenzone (OB) - ε = 15150 at 287 nm;
- 2-ethylhexyl-4-methoxy cinnamate (OMS) - ε = 39470 at 1356 nm;
- Avobenzon - ε = 31670 at 310 nm.
Comparing these indicators with experimental ones, we can say with confidence that the studied compounds have very good UV absorbing properties.
For a more detailed acquaintance with the nuances of the study, I recommend that you look into the report of scientists.
Epilogue
Use with the sense that in the opinion of the majority is of no use is always good. If we also take into account the fact that oil products are not endless, and their impact on the environment is far from positive, then the creation of substitutes from biomaterials is becoming one of the alternative ways for humanity. Cashew nut husk sunscreen, i.e. based on biomass, this is only the initial stage of the study of what other "useless" substances have hidden beneficial properties and practical applications. After all, everything has an application, just sometimes we don’t know which one.
Thank you for your attention, remain curious and have a good working week, guys! :)
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending it to your friends, a 30% discount for Habr users on a unique analogue of entry-level servers that we invented for you: The whole truth about VPS (KVM) E5-2650 v4 (6 Cores) 10GB DDR4 240GB SSD 1Gbps from $ 20 or how to divide the server? (options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Dell R730xd 2 times cheaper? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands! Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - from $ 99! Read about How to Build Infrastructure Bldg. class c using Dell R730xd E5-2650 v4 servers costing 9,000 euros for a penny?
All Articles