Gene Therapy Liz Parrish - An Attempt to Understand
In the light of my dreams of gene therapy with the help of Yamanaki factors , I decided to brush up on the details of the self-experiment Liz Parrish. Suddenly she agrees to act as the first patient for Yamanach therapy? No, I'm joking, of course, but there is some seriousness in this joke. I really think that gene therapy for epigenetic recoil has therapeutic potential. And for some $ 15–20 million, this potential can either be brought to the start of clinical trials, or refuted.
Well, okay, coming back from heaven to earth - what did Liz Parrish introduce herself to? According to her, Liz introduced herself 2 different gene therapies using adeno-associated viral (AAV) vectors: the hTERT telomerase gene and the follistatin FS gene (designed to inhibit myostatin).
It is necessary to clarify that, most likely, it was not two types of AAV, but much more, since Liz needed to prepare a specific AAV for each type of target tissue. And then she had to deliver this particular AAV to this target tissue. This is what I understood from this Liz interview on Longecity Now:
http://www.longecity.org/media/Liz_Parrish_LongeCity_Now2016.mp3
It is worth noting that AAV is not purposefully integrated into the genome - that is, for those cells that divide, the daughter cells will NOT receive those genes that were delivered to the original cell using AAV. But they will have elongated telomeres, provided that the hTERT gene works as intended in their mother cell:
https://www.addgene.org/viral-vectors/aav/aav-guide/
The fact that Liz actually injected herself with some kind of injection (yes, skeptics, I hear you) is confirmed by the director of a documentary film about her that filmed this procedure in Colombia:
By the way, according to Liz, AAV therapy successfully delivered the hTERT telomerase gene to only 20% of its cells. She stated this at her presentation at Digital October in Moscow, June 22, 2016 (at about 1:46:41 of this video):
Why did Liz choose these particular treatments? About the rejuvenating potential of telomerase, Michael Fozsel and Bill Andrews have been talking for many years. The specific approach of applying TERT-therapy was confirmed by Maria Blasco in mice, where she extended both average survival and maximum age of mice in two groups - one received TERT injections at the age of 420 days (increase in median survival by 24% and increase by 13% in maximum life expectancy), and another at the age of 720 days (an increase in median survival by 20% and an increase in maximum life expectancy by 13%):
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3494070/
By the way, Michael Fozsel said that he had consulted Liz several times, as he, as he works on the beginning of clinical trials of a very similar approach of hTERT therapy for Alzheimer's patients:
As for the second Liz therapy using the follistatin gene, then, as I understand it, this approach was previously tested on itself by partner Liz and co-owner of BioViva, Jason Williams. Williams, by the way, has developed several other experimental AAV gene therapies before, and also has his own clinic in Colombia, where he offers some of these therapies to patients (but Liz did not go through her procedures in his clinic):
http://www.neuralgene.com/technology-pipeline.cfm
The specific FS AAV therapy that Liz has introduced herself has already successfully completed the second phase of CI in Britain, and is now undergoing the third:
http://www.nature.com/mt/journal/v23/n1/full/mt2014200a.html
Question: Why test two different treatments at the same time? Liz says that she expects a synergistic effect, and also that there is evidence that FS therapy can deplete stem cells, and hTERT therapy should help prevent this.
The first results of the treatment reported by BioViva are that the telomeres of Liz's lymphocytes were lengthened from 6,710 to 7,330 base pairs after treatment. The statement that this is equivalent to “rolling back telomeres 20 years ago”, although it sounds pathetic, but has some scientific justification, since the average speed of leukocyte telomeres shortening is about 30 bases of pairs per year:
At the same time, some people note that telomere elongation is within 8–10% of the measurement error of qPCR, but from what I read about qPCR, 8–10% is an interlaboratory or intermethod error, and not an error when using same equipment in the same lab. That is, you can see a 10% difference in the results when testing the same sample in two different laboratories or using two different methods. But Liz claims that her telomeres were measured both before and after receiving treatment in the same laboratory - SpectraCell Laboratories.
In addition, Liz argues that the results were confirmed by two other scientific organizations: the Belgian NGO HEALES and the British Foundation for Biogerontological Research. Therefore, the probability that the difference of 9% is due to measurement errors seems to me much less than the probability that this difference is due to real biological changes. Of course, for greater certainty, it would be necessary to analyze all the samples of Liz and other methods (not qPCR, but TRF, for example), and in other laboratories. Hope it will be done.
By the way, I have to note that it remains an open question how independent the above-mentioned scientific organizations are, since HEALES is associated with the ILA (International Longevity Alliance), where Liz is a member of the board of directors, and the British Foundation for Biogerontological Research is headed by Avi Roy, who is also Director of Science at BioViva.
By the way, at a press conference in Moscow, Liz said that she sent some of her samples before and after the therapy to the George Church laboratory at Harvard, which promised to carry out their detailed analysis, including tests evaluating methylation changes (hoping to see " more young "profile of methylation hours , identified in the works of Steve Horvath). Liz talks about this in the same video (around 1:48:41):
George Church is, of course, cool, because he is almost Einstein of modern genetics, but ... he is on the BioViva Scientific Council. Hopefully, over time, there will be a completely independent third party who will undertake to confirm the results of Liz, preferably using another telomere measurement method. At the same press conference, Liz said that she was ready to provide her samples for testing in independent laboratories, provided that they were truly authoritative.
In March 2017, Liz released a press release with her latest test results:
https://bioviva-science.com/blog/2017/3/2/dual-gene-therapy-has-beneme-effects-on-blood-biomarkers-and-muscle-composition
There, she claims that MRI data of her thighs show a decrease in the “marbling” of the muscles, that is, a decrease in the amount of intramuscular fat deposits, which can be regarded as a positive effect from follistinic therapy:
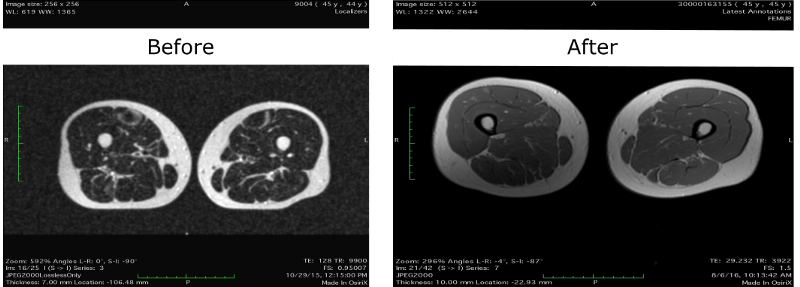
But, perhaps, the visual difference between the images is due only to an improvement in the resolution of the MRI: the old image was taken on the device in 1 Tesla, and the new one - 1.5 Tesla. In addition, it seems to me that the images were taken from slightly different places in the hips - more recent images, judging by the distance between the legs, were taken from sections slightly lower from the pelvis than the old ones. And the lower the priests, the lower the percentage of fat in the legs. Although, maybe, Liz just spread her legs a little wider in the last test.
By the way, in the recent data Liz was interested to see a rather high (1.6) level of C-reactive protein before the introduction of gene therapy and the subsequent decrease in CRP to 0.2 in February 2016. It would be interesting to know what the value of CRP was in the test round in 2016 - Liz cites glucose and triglyceride values from August 2016, but not the CRP.
In conclusion, I will mention a couple of critical comments on the use of telomere-length lymphocytes as markers of “rejuvenation”:
Under paragraph (A), Michael Vossell wrote a detailed post on his blog, so I’ll just give him a link:
http://www.michaelfossel.com/blog/?p=182
And under (B), yes, the average length of telomeres can, apparently, sometimes spontaneously increase, although not as much as in the case of Liz, and this happens infrequently (in 10-15% of cases). Here are two good papers on the subject:
http://www.clinsci.org/content/128/6/367.full
https://www.hindawi.com/journals/jir/2016/5371050/
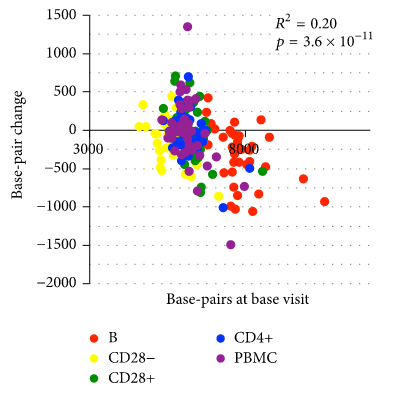
This graph from the last work is especially indicative - as we can see, in 18 months for quite a few people the telomeres lengthened (all points above zero):
By the way, some hardcore skeptics require evidence that Liz really introduced herself to the stated genes. In principle, this could be cheaply and quickly tested using RT-qPCR on Liz lymphocytes before and after therapy: if AAV delivered its genes, we will have to see the difference in the curves. Perhaps George Church’s lab could do this analysis. But I do not think it is necessary, because frankly lying about introducing these genes to yourself, and then sending your samples to George Church would be the height of idiocy.
Well, okay, coming back from heaven to earth - what did Liz Parrish introduce herself to? According to her, Liz introduced herself 2 different gene therapies using adeno-associated viral (AAV) vectors: the hTERT telomerase gene and the follistatin FS gene (designed to inhibit myostatin).
It is necessary to clarify that, most likely, it was not two types of AAV, but much more, since Liz needed to prepare a specific AAV for each type of target tissue. And then she had to deliver this particular AAV to this target tissue. This is what I understood from this Liz interview on Longecity Now:
http://www.longecity.org/media/Liz_Parrish_LongeCity_Now2016.mp3
It is worth noting that AAV is not purposefully integrated into the genome - that is, for those cells that divide, the daughter cells will NOT receive those genes that were delivered to the original cell using AAV. But they will have elongated telomeres, provided that the hTERT gene works as intended in their mother cell:
https://www.addgene.org/viral-vectors/aav/aav-guide/
The fact that Liz actually injected herself with some kind of injection (yes, skeptics, I hear you) is confirmed by the director of a documentary film about her that filmed this procedure in Colombia:
MIT Technology Review tried to confirm aspects of Parrish’s story by talking to Matthew Andrews, a Los Angeles-based film director who said that he shot Parrish’s treatment in September — it was a modest doctor’s office where one doctor and one nurse were present who also collected blood tests . “It was a treatment room, there were no particularly high-tech gadgets. She was lying on the bed, without anesthesia, receiving injections and connected to an IV line, ”he said. “It was boring from an observer’s point of view, although I certainly don’t know what was going on inside the body.
https://www.technologyreview.com/s/542371/a-tale-of-do-it-yourself-gene-therapy/
By the way, according to Liz, AAV therapy successfully delivered the hTERT telomerase gene to only 20% of its cells. She stated this at her presentation at Digital October in Moscow, June 22, 2016 (at about 1:46:41 of this video):
Why did Liz choose these particular treatments? About the rejuvenating potential of telomerase, Michael Fozsel and Bill Andrews have been talking for many years. The specific approach of applying TERT-therapy was confirmed by Maria Blasco in mice, where she extended both average survival and maximum age of mice in two groups - one received TERT injections at the age of 420 days (increase in median survival by 24% and increase by 13% in maximum life expectancy), and another at the age of 720 days (an increase in median survival by 20% and an increase in maximum life expectancy by 13%):
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3494070/
By the way, Michael Fozsel said that he had consulted Liz several times, as he, as he works on the beginning of clinical trials of a very similar approach of hTERT therapy for Alzheimer's patients:
Our biotechnology firm, Telocyte, intends to do almost the same thing, but with a number of important differences: we will use only one therapy - therapy with the telomerase gene (hTERT), and we intend to conduct comprehensive tests in humans, having received FDA approval, with the procedure aligning the IRB, as well as using GMP therapy production standards.
In doing so, I applaud Liz for courage to use myself as a research subject. Using herself as a subject removes much of ethical criticism, which would be more relevant if Liz had used other patients. Like many others, we see the urgent need to develop and implement more effective therapeutic interventions: patients not only suffer, but also die, while we are trying to move forward. In the case of Alzheimer's disease, for example (our main therapeutic target for Telocyte), unfortunately, there are currently no effective treatments, and a huge population of patients is forced to die while we are developing a new therapy for their treatment. A slow, balanced approach to finding a treatment is hardly welcomed by them in such a situation.
But still…
We decided to follow a standard approach — with FDA approval for human testing — for three reasons that we consider important: 1) we want to ensure safety, 2) we want to be effective, and 3) we want to provide credibility. The issue of safety is not simple: Alzheimer's disease is fatal, so safety may seem less important here than effectiveness. And we really believe that there is no reason not to try experimental therapy on desperate patients, if you remove the easily avoidable risks in advance (for example, using safe production processes for viral vectors). The question of efficiency is also not easy: someone says that one should try any kind of terpapy, even if remotely effective. But we see no reason to use minimally effective therapy if we can provide the most effective therapy using only a little more foresight and care. The question of trust is also not simple: someone claims that it will be enough just to cure at least one patient of Alzheimer's disease. This is true, but only on the condition that we believe that we really cured him. But if no one believes us, the fact that we have cured someone alone will not help millions of other patients.
http://www.michaelfossel.com/blog/?p=139
As for the second Liz therapy using the follistatin gene, then, as I understand it, this approach was previously tested on itself by partner Liz and co-owner of BioViva, Jason Williams. Williams, by the way, has developed several other experimental AAV gene therapies before, and also has his own clinic in Colombia, where he offers some of these therapies to patients (but Liz did not go through her procedures in his clinic):
http://www.neuralgene.com/technology-pipeline.cfm
The specific FS AAV therapy that Liz has introduced herself has already successfully completed the second phase of CI in Britain, and is now undergoing the third:
http://www.nature.com/mt/journal/v23/n1/full/mt2014200a.html
Question: Why test two different treatments at the same time? Liz says that she expects a synergistic effect, and also that there is evidence that FS therapy can deplete stem cells, and hTERT therapy should help prevent this.
The first results of the treatment reported by BioViva are that the telomeres of Liz's lymphocytes were lengthened from 6,710 to 7,330 base pairs after treatment. The statement that this is equivalent to “rolling back telomeres 20 years ago”, although it sounds pathetic, but has some scientific justification, since the average speed of leukocyte telomeres shortening is about 30 bases of pairs per year:
With normal aging, telomeres are shortened in CD4 + T-helpers, CD8 + cytotoxic T-cells, and antibody-producing B-cells at an annual rate of 19–35 base pairs
http://www.ncbi.nlm.nih.gov/pubmed/10903716
http://www.ncbi.nlm.nih.gov/pubmed/12437664
At the same time, some people note that telomere elongation is within 8–10% of the measurement error of qPCR, but from what I read about qPCR, 8–10% is an interlaboratory or intermethod error, and not an error when using same equipment in the same lab. That is, you can see a 10% difference in the results when testing the same sample in two different laboratories or using two different methods. But Liz claims that her telomeres were measured both before and after receiving treatment in the same laboratory - SpectraCell Laboratories.
In addition, Liz argues that the results were confirmed by two other scientific organizations: the Belgian NGO HEALES and the British Foundation for Biogerontological Research. Therefore, the probability that the difference of 9% is due to measurement errors seems to me much less than the probability that this difference is due to real biological changes. Of course, for greater certainty, it would be necessary to analyze all the samples of Liz and other methods (not qPCR, but TRF, for example), and in other laboratories. Hope it will be done.
By the way, I have to note that it remains an open question how independent the above-mentioned scientific organizations are, since HEALES is associated with the ILA (International Longevity Alliance), where Liz is a member of the board of directors, and the British Foundation for Biogerontological Research is headed by Avi Roy, who is also Director of Science at BioViva.
By the way, at a press conference in Moscow, Liz said that she sent some of her samples before and after the therapy to the George Church laboratory at Harvard, which promised to carry out their detailed analysis, including tests evaluating methylation changes (hoping to see " more young "profile of methylation hours , identified in the works of Steve Horvath). Liz talks about this in the same video (around 1:48:41):
George Church is, of course, cool, because he is almost Einstein of modern genetics, but ... he is on the BioViva Scientific Council. Hopefully, over time, there will be a completely independent third party who will undertake to confirm the results of Liz, preferably using another telomere measurement method. At the same press conference, Liz said that she was ready to provide her samples for testing in independent laboratories, provided that they were truly authoritative.
In March 2017, Liz released a press release with her latest test results:
https://bioviva-science.com/blog/2017/3/2/dual-gene-therapy-has-beneme-effects-on-blood-biomarkers-and-muscle-composition
There, she claims that MRI data of her thighs show a decrease in the “marbling” of the muscles, that is, a decrease in the amount of intramuscular fat deposits, which can be regarded as a positive effect from follistinic therapy:

But, perhaps, the visual difference between the images is due only to an improvement in the resolution of the MRI: the old image was taken on the device in 1 Tesla, and the new one - 1.5 Tesla. In addition, it seems to me that the images were taken from slightly different places in the hips - more recent images, judging by the distance between the legs, were taken from sections slightly lower from the pelvis than the old ones. And the lower the priests, the lower the percentage of fat in the legs. Although, maybe, Liz just spread her legs a little wider in the last test.
By the way, in the recent data Liz was interested to see a rather high (1.6) level of C-reactive protein before the introduction of gene therapy and the subsequent decrease in CRP to 0.2 in February 2016. It would be interesting to know what the value of CRP was in the test round in 2016 - Liz cites glucose and triglyceride values from August 2016, but not the CRP.
In conclusion, I will mention a couple of critical comments on the use of telomere-length lymphocytes as markers of “rejuvenation”:
- (A) telomere measurement of lymphocytes is not a good metric in principle, since they have too much variation in telomere length
- (B) the average length of telomeres, especially in white blood cells (WBC), can fluctuate naturally over a fairly short period of time (less than 2 years)
Under paragraph (A), Michael Vossell wrote a detailed post on his blog, so I’ll just give him a link:
http://www.michaelfossel.com/blog/?p=182
And under (B), yes, the average length of telomeres can, apparently, sometimes spontaneously increase, although not as much as in the case of Liz, and this happens infrequently (in 10-15% of cases). Here are two good papers on the subject:
http://www.clinsci.org/content/128/6/367.full
https://www.hindawi.com/journals/jir/2016/5371050/
This graph from the last work is especially indicative - as we can see, in 18 months for quite a few people the telomeres lengthened (all points above zero):

By the way, some hardcore skeptics require evidence that Liz really introduced herself to the stated genes. In principle, this could be cheaply and quickly tested using RT-qPCR on Liz lymphocytes before and after therapy: if AAV delivered its genes, we will have to see the difference in the curves. Perhaps George Church’s lab could do this analysis. But I do not think it is necessary, because frankly lying about introducing these genes to yourself, and then sending your samples to George Church would be the height of idiocy.
All Articles