
Any system, no matter how complex and multi-layered it may be, has its own foundation - the basis without which it would not work the way it works. In the biosphere of our planet there are also basic bricks on which everything rests. They are autotrophs - organisms that can convert inorganic compounds into organic ones. Today we will meet with you a study in which scientists from Israel created in the laboratory a new type of bacteria that feed on carbon dioxide. What methods were used in the development process, how did the bacterium behave, and what can this work mean for humanity? We learn about this from the report of the research group. Go.
Study basis
Autotrophs can be called one of the most ancient creatures on the planet. It is believed that the first autotrophs appeared two billion years ago, when a heterotrophic (unable to synthesize organics from inorganic) bacterium through evolution acquired the ability to photosynthesis. The term itself was proposed back in 1892 by the German scientist Albert Bernhard Frank.

Albert Bernhard Frank
Some organisms are at the crossroads of autotrophic and heterotrophic synthesis, since they get carbon from organic compounds, but energy from inorganic ones. Following this logic, autotrophs can be divided into several main ones: phototrophs, chemotrophs, radiotrophs, lithotrophs, and mixotrophs. There are also transitional groups, the representatives of which are extremely difficult to attribute to one or another edge of the synthesis spectrum, but their classification has not yet been completed.
Phototrophs , as the name implies , use photons as a source of energy, or rather solar energy. It is from these organisms that such a type of nutrition as photosynthesis occurs.
Chemotrophs are closer chemistry than physics. Such organisms use various redox reactions as energy sources, i.e. chemosynthesis.
The most poorly studied and at the same time the most curious type are radiotrophs - the result of a phenomenon called "radio stimulation of mushrooms." This is the process of stimulating the microscopic fungal metabolism due to ionizing radiation. For the first time, these organisms were found back in 1991 as part of black mold, samples of which were obtained from the Chernobyl nuclear power plant. A little later (in 2006), scientists from New York tested the hypothesis of radio stimulation of fungi and found that three fungi of the species Cladosporium sphaerospermum, Wangiella dermatitidis, and Cryptococcus neoformans, which contain the pigment melanin, increased their biomass and accumulated acetate (acetic acid, 3CHOO ) in an environment where the radiation level exceeded the norm by 500 times.
Lithotrophs process inorganic compounds into the energy and carbon they need through aerobic or anaerobic respiration. Only representatives of archaea (unicellular without a nucleus and membrane organelles) and bacteria can boast of chemolithotrophy.
Mixotrophs are universal soldiers, because they can use several different types of food at the same time (or alternately, depending on the circumstances), i.e. be both phototrophs and chemotrophs, for example.
The authors of the study we are considering today believe that a more detailed understanding of the processes of vital activity of autotrophs will allow applying the acquired knowledge on a large scale. And in their opinion, the best way to study autotrophy is to create a synthetic autotrophic organism. Theoretically, you can create a bacterium that will feed on carbon dioxide. But to call this process easy language does not turn. Researchers themselves identify three main stages that must be completed in order for their work to be realized.
Firstly, for a complete transition to autotrophic nutrition, the body must use the mechanism of CO 2 fixation in the path where the incoming carbon consists exclusively of CO 2 , and the output molecules are organic molecules that enter into central carbon metabolism and supply all 12 major biomass precursors.
Secondly, the body must use enzymatic mechanisms to obtain reducing power by collecting non-chemical energy (light, electricity, etc.) or by redox processes that are not carbon sources.
Thirdly, the body must regulate and coordinate the ways of collecting energy and fixing CO2, so that they together maintain steady growth when CO2 is the only source of carbon.
Earlier, studies were conducted in which they tried to create an organism that feeds on CO 2 , but in those works there was one big flaw - the presence of multi-carbon organic compounds inside the body, which served as a "reserve" source of nutrition. In other words, it has not yet been possible to create a heterotrophic organism that will take carbon exclusively from CO 2 .
As the methodological basis of their study, scientists used the Calvin cycle (Calvin-Benson-Bassam cycle) - a series of biochemical reactions during photosynthesis in plants, cyanobacteria, etc. This cycle is the most common carbon dioxide fixation mechanism.
And the main test was the bacterium Escherichia coli , better known to us under the name "E. coli."
Research results
First of all, it was necessary to carry out metabolic reorganization and laboratory evolution of the body in order to realize the transition to autotrophy. Several candidate compounds were considered that could serve as electron donors for the fixation of CO 2 , which would allow the bacteria to transfer to complete autotrophy.
Formate * was chosen as the electron source, since this one-carbon organic compound can serve as a source of the reducing part of the process, but does not naturally support E. coli growth and is not absorbed into biomass.
Formates * - salts and esters of formic acid.The recovery potential of formate (E 0 = 420 mV) is low enough to reduce NAD + , the main electronic carrier in the cell (E 0 = 280 mV in E. coli ). Another advantage is that it can be electrochemically obtained from renewable sources, while the biomass will be carbon-negative.
In order to collect electrons from formate and direct them to the NADH (Niacinamide Adenine Dinucleotide) main cellular energy recovery reservoir, NAD + -linked FDH (formate dehydrogenase) from the methylotrophic bacterium Pseudomonas sp .
A stoichiometric analysis (mass ratio of the chemical compound) of the metabolic network in E. coli showed that adding FDH, Rubisco (ribulose bisphosphate carboxylase) and Prk (phosphoribulokinase) to the E. coli metabolic network would be sufficient for autotrophic growth (image below).

Image No. 1: Scheme of a laboratory-modified chemotrophic bacterium E. coli.
Unfortunately, coexpression of three recombinant enzymes in the primary E. coli strain (BW25113) did not lead to growth under autotrophic conditions. Since stoichiometric analysis does not take into account the kinetics of the enzyme, the level of expression and regulation, it was decided to use adaptive laboratory evolution as a tool for metabolic optimization to achieve autotrophic growth.
This method is due to the fact that heterologous expression of a foreign enzymatic mechanism expands the space of possible metabolic reactions for the cell, providing the possibility of autotrophic growth. The problem is that there is no guarantee that the necessary stream will go through a recently expanded set of reactions.
Therefore, since the central metabolism of E. coli is adapted to heterotrophic growth, it is likely that a flow distribution that supports heterotrophic growth will be used. That is precisely why laboratory evolution was used, capable of redirecting the flow along the desired metabolic pathway.
One of the most important processes of laboratory evolution is the rearrangement of central metabolism to establish dependence on the Rubisco carboxylation stream and the adaptation of the growth medium to suppress the flow through the original heterotrophic pathways ( 2A ). In other words, it was necessary to make the bacterium stop using the heterotrophic mechanisms of metabolism, switching to autotrophy.

Image No. 2: scheme of the developed evolutionary strategy for converting the heterotrophic bacterium E. coli into a chemotrophic one.
First of all, during the artificial evolution, three genes encoding two enzymes in central carbon exchange were excluded: phosphofructokinase (Pfk) in glycolysis and glucose-6-phosphate dehydrogenase (Zwf) in the oxidative pentose phosphate pathway. The first has two isoenzymes encoded by two genes (pfkA and pfkB). When cells are grown on xylose *, this rearrangement ensures that cell growth depends on Rubisco carboxylation, which is necessary for the transition to chemotrophy.
Xylose * is a pentose monosaccharide (C 5 H 10 O 5 ).Next, heterologous expression of Rubisco, Prk, carbonic anhydrase (CA) was carried out, which converts CO 2 and bicarbonate and FDH. Following this is the process of growing cells in xylose-limited chemostats * , which support cells in constant carbon starvation.
Chemostat * is a method of cultivating microorganisms when the optimal balance and concentration of substrates is maintained in the nutrient medium where they grow.Such a culture medium allows cells to proliferate (tissue growth by cell division), but slows down heterotrophic catabolic * pathways.
Catabolism * - metabolic decomposition of complex substances into simpler ones or oxidation of a substance (energy metabolism).The chemostat, where the cells were grown, also contained an excess of formate and was constantly purged with CO 2 enriched air (CO 2 content of 10%).
Thus, this growth medium slows down heterotrophy, causing cells to tend to autotrophy. Cells are literally forced to reduce their dependence on the external carbon contribution of organic sugar.
The growing method was ready, it was necessary to verify it. Once a week, samples were removed from chemostats and tested for growth under autotrophic conditions. In particular, these are chemo-organotrophic conditions for Escherichia coli, which consist of a medium of type M9 with the addition of 30 mM (millimolar) sodium formate in an atmosphere with a high content of CO 2 (10%), but without any other carbon source.
After approximately 200 days of propagation in chemostats, equivalent to approximately 150 generations, growth was detected in media lacking xylose (i.e., under autotrophic conditions). This phenotype was present in all samples of that day. On the 350th day, xylose was completely excluded from the culture medium ( 2B ). Sustainable growth and turbidity implied that exclusively xylose-independent cells were present in the chemostat. It was found that the samples needed a medium with a high concentration of CO 2 for their growth, which suggests a carbon fixation mechanism.
Next, scientists chose one of the most robust clones in growth * for a deeper analysis. The doubling time was established * 18 ± 4 hours ( 2C ).
Clone * - in this case, we mean a group of genetically identical cells.
Doubling time * - the time it takes for something to double in size.It was necessary to make sure that the grown cells are really autotrophic, and during their growth there were no “hidden” carbon sources or heterotrophic formate activation. To do this, experiments were conducted on the labeling of isotopes.
To begin with, the evolved clones were grown in a medium with 13 C-labeled formate and 13 CO 2 (10 generations until a stable isotopic state was obtained). Next, an analysis of 13 C-labels for various metabolites was carried out by liquid chromatography and tandem mass spectrometry.
13 C * - carbon-13, a stable carbon isotope.
Metabolites * - metabolic products of any compounds.
The method of liquid chromatography and tandem mass spectrometry * is a chemical research method that combines liquid chromatography separating mixtures of several components and mass spectrometry, which ensures the structural identity of the individual components.
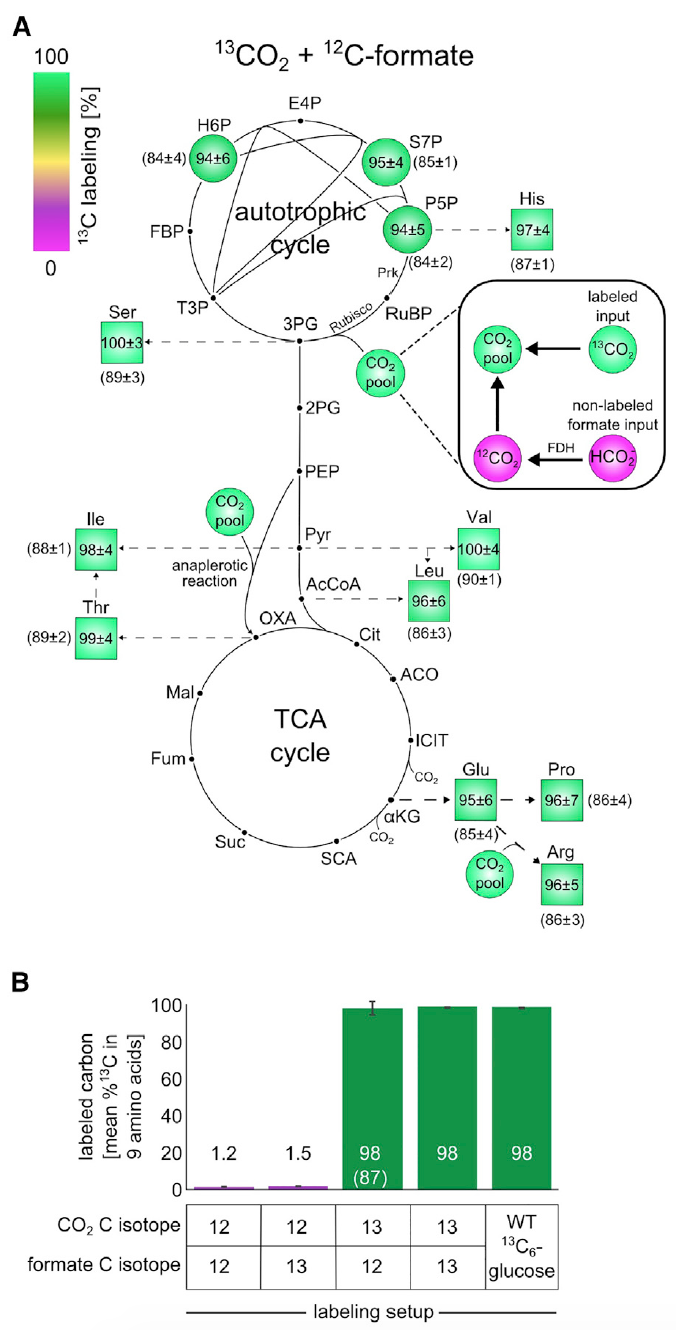
Image 3: Isotope labeling experiments using 13 C show that all biomass components are formed from CO 2 as the sole carbon source.
The analysis showed that about 98% of the carbon atoms in the building blocks of the central metabolism biomass were successfully labeled. These data correspond to labeled formate and CO 2 containing about 99% 13 C and 1% unlabeled bicarbonate dissolved in a nutrient medium.
This observation is irrefutable evidence that the carbon in the biomass of cells comes precisely from CO 2 and formate.
Further, it was checked whether formate is concentrated in biomass. For this, cells were grown in M9 medium (the concentration of CO 2 in the atmosphere was 10%, as in previous experiments) using formate labeled with carbon-13.
The 13 C marking scheme for building blocks of biomass after growth in a given environment showed a 13 C mark within 1-2% ( 3B ), which is the expected value based on the natural 13 C content and a small amount of labeled formate. In other words, the results showed that cells do not assimilate formate.
The totality of the results of the above experiments confidently suggests that the source of carbon for the grown crops are exclusively CO2 and formate. And this, in turn, indicates a one hundred percent autotrophy of E. coli cells that have undergone laboratory evolution.
Scientists conducted another experiment to verify this statement, where labeled 13 CO 2 and unlabeled formate were used. Due to the high cost of 13 CO 2 , the vessels in which the experiment was conducted were closed. This small nuance is extremely important, because due to the closed environment (in the previous experiments, the containers were ventilated), unlabelled CO 2 accumulated due to the oxidation of formate was accumulated. And this distorts the observation results. However, the “contamination” process could be tracked and even corrected with an eye on it through analysis of labeled glutamate.
This experience showed that about 85-90% of the carbon atoms in the building blocks of the central metabolism biomass were successfully labeled. As can be seen in images 3A and 3B , if we apply the adjustment for the 13 C-labeled component, then the labeling of atoms in the biomass will be almost 100%, which indicates the autotrophic nature of the development of E.coli bacteria.
That bacteria have become autotrophs is beyond doubt. It remains to find out what kind of genetic changes, that is, mutations, occurred in the process of laboratory evolution.
To clarify this, scientists isolated six clones capable of autotrophic growth on formate and sequenced their genome and plasmids * .
Plasmids * are DNA molecules physically separated from chromosomes and capable of autonomously replicating (the process of creating two daughter DNA molecules based on the parent DNA molecule).Two clones (clone 1 and 2) were isolated when xylose was still present in the medium (250th day of evolution), three clones (clone 3, 4 and 5) after xylose was excluded from the medium of the chemostat ( 400th day of evolution). The last clone (clone 6) is isolated after the propagation of one of the previously isolated clones (clone 1) for several series dilution cycles.
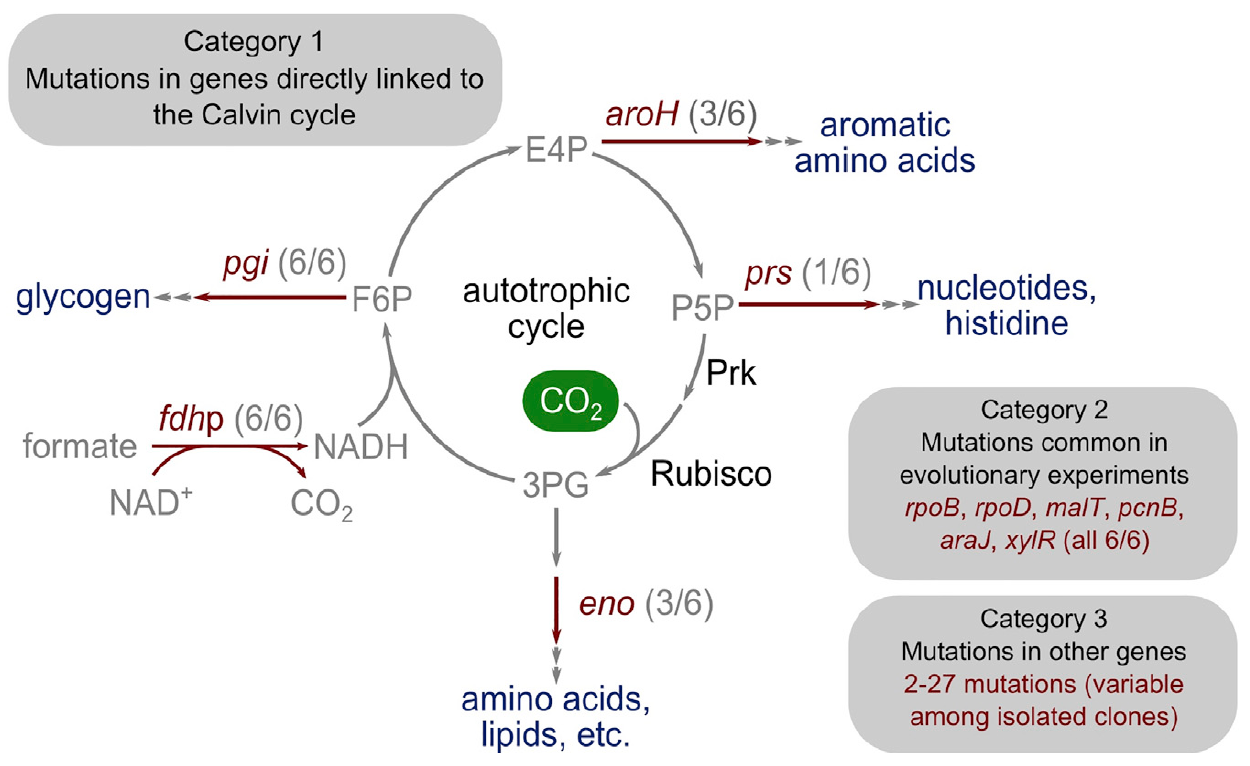
Image 4: The genetic basis of the transition to autotrophy.
Surprisingly, the number of mutations was quite small. Researchers divided them into three main categories.
The first category consists of genes encoding enzymes with a direct metabolic link with the function of the Calvin cycle. This gene, which encodes ribose phosphate diphosphokinase, directs ribose phosphate to biomass.
The second category of mutated genes consists of those that mutated in previous experiments on adaptive laboratory evolution: pcnB (R161P), rpoB (D866E), rpoD (F563S), malT (E359K) and araJ (W156). Scientists associate these mutations with the very process of laboratory evolution, that is, they are not necessarily associated with the process of the transition of bacteria to autotrophy. Similarly, a mutation in the xylR gene was found that encodes a regulatory protein for operons responsible for the catabolism of D-xylose sugar (E337K). It is associated with prolonged starvation of xylose in a chemostat during cultivation, but is in no way associated with autotrophy.
The third category of mutations includes those that do not have a characteristic role and may be the result of such a phenomenon as “genetic hitchhiking”. In different isolates, there are anywhere from 2 to 27 additional mutated genes, some of which may be mutations of the autotrophic phenotype, but are not strictly necessary for it.
In the future, scientists intend to conduct additional studies of genetic mutations to determine which of them are basic and necessary for the transition of bacteria to autotrophy.
For a more detailed acquaintance with the nuances of the study, I recommend that you look into the report of scientists and additional materials to it.
Epilogue
In this study, scientists were able to achieve excellent results. First of all, it is worth noting laboratory evolution - a process controlled by scientists, allowing them to transform the body according to a new "design".
E. coli was literally forced by evolutionary manipulation to become an autotroph, absorbing CO 2 to provide itself with carbon. Such a mutant bacterium can be extremely useful for a society suffering from global warming, one of the reasons for which is carbon dioxide. However, as the researchers themselves admit, at the moment their bacterium produces more CO 2 during the absorption of formate than it absorbs during carbon fixation. Despite this “awkward” situation, it would be a crime to consider this work of scientists useless. Firstly, these are only the first steps in understanding the transformation of heterotrophic organisms into autotrophic. Secondly, further studies will make it possible to choose a more efficient energy source for bacteria than formate, which will significantly reduce the release of CO 2 . The main thing is that the foundation has already been laid, it remains only gradually and very carefully to insert brick by brick.
The bacterium that will save the world from global warming, it sounds incredible, even a little sci-fi. But, even if such a bacterium is created, this does not relieve a person of responsibility for the environmental situation on the planet. For it’s purely not where they clean, but where they do not litter.
Thank you for your attention, remain curious, take care of the planet and have a good working week, guys. :)
A bit of advertising :)
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to your friends cloud-based VPS for developers from $ 4.99 , a unique analog of entry-level servers that was invented by us for you: The whole truth about VPS (KVM) E5-2697 v3 (6 Cores) 10GB DDR4 480GB SSD 1Gbps from $ 19 or how to divide the server? (options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).
Dell R730xd 2 times cheaper at the Equinix Tier IV data center in Amsterdam? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands! Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - from $ 99! Read about How to Build Infrastructure Bldg. class c using Dell R730xd E5-2650 v4 servers costing 9,000 euros for a penny?