A lead-acid battery cannot be called a miracle of modern engineering. It is very reliable and easy to use, and to charge it you just need to apply a fixed voltage to it and wait a bit; as a result, the battery charges and remains fully charged - that’s it. On the other side of this simplicity are their size, weight, energy density and toxicity of materials.
A lithium battery is a modern hit, however, its high energy density leads to the fact that its small case can become angry and become very dangerous if mistreated. Scientists are looking for safer battery options, improved charging systems, formulas to extend battery life that could be recharged thousands of times, and one recent publication has caused a lot of enthusiastic responses.
Consider the requirements for the battery cell in an electric vehicle:
- High energy density (large amount of energy in a small battery).
- The ability to quickly charge.
- Possibility of quick discharge.
- LOTS of charge / discharge cycles.
- Low self discharge.
- Security.
At the moment, lithium-ion batteries are the best option, but there are plenty of chemical reactions with lithium, and depending on the planned use, balancing and charging, it is possible to optimize various reaction variants for different operating characteristics. While there is no perfect battery, and conflicting demands guarantee the availability of several options on the market.
How lithium + ion works
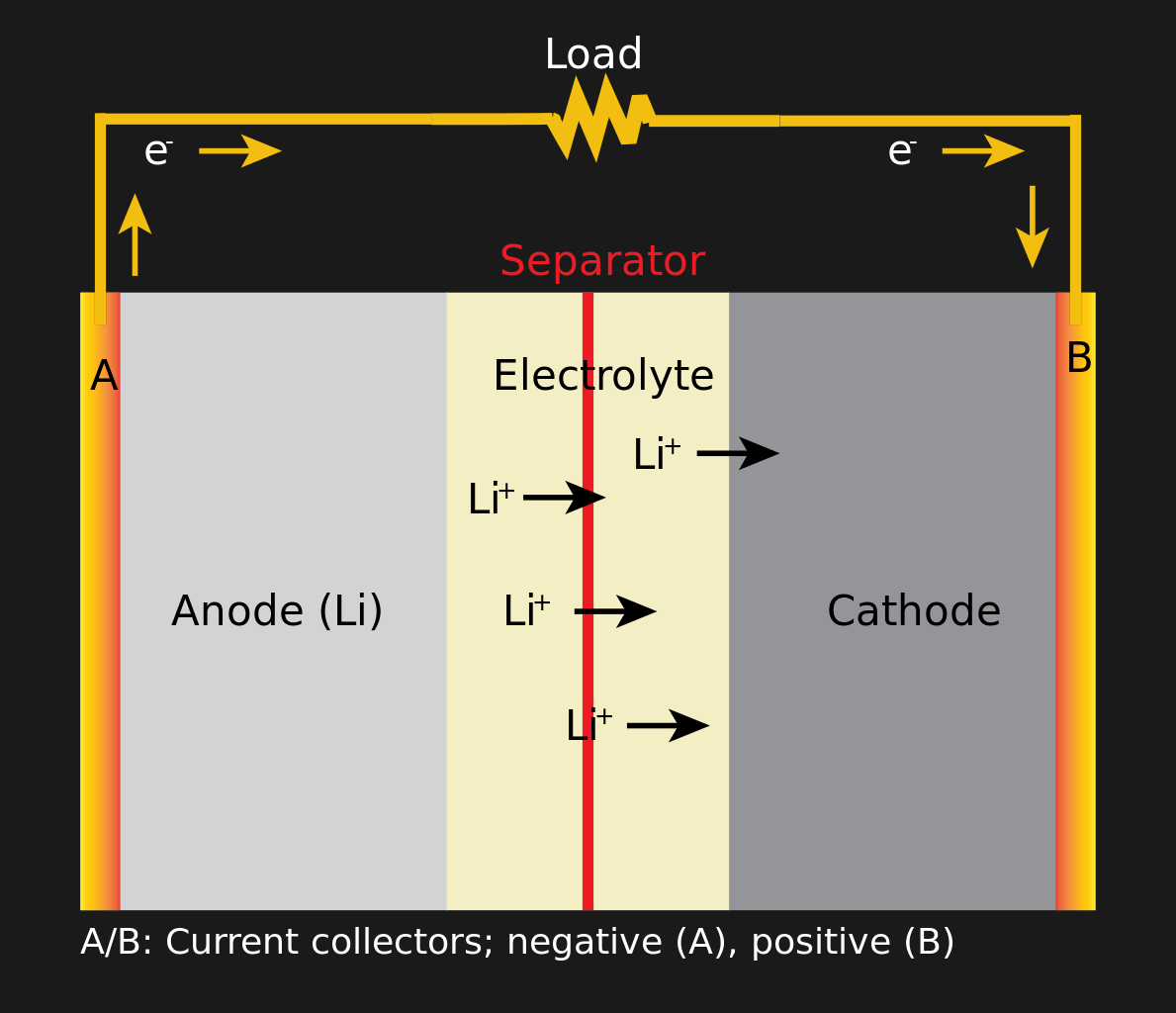
Discharging a Li-ion Battery
All batteries work the same. There are three components: anode, cathode and electrolyte. A chemical reaction between an electrolyte and electrodes (anode and cathode) creates ions near one electrode and electrons next to another, resulting in a potential difference. Pairs of electrodes are made of different materials. The anode is graphite bonded to copper, and the cathode is a lithium crystal bonded to aluminum. The electrolyte works like an insulator, so it is easier for electrons to go from one electrode to another through a circuit than inside the battery. At the end of the reaction, the battery discharges, and the reaction will no longer go if the electrons have nowhere to go. To charge the battery, the process reverses, and the voltage supplied to the electrolyte starts the reaction in the opposite direction. Not all electrolytes are created equal; The chemistry of the battery, which cannot be recharged, allows it to store more energy, but the application of reverse voltage in it does not start the chemical reaction back.
The capabilities of the battery are best revealed by increasing the surface area of the electrodes, so it is better to make a sandwich from the anode, electrolyte and cathode as thin as possible and with a larger contact area. The sandwich also includes several slices of other porous materials that allow ions to pass through but prevent materials from migrating. Take a few of your battery sandwiches and stack them together, interspersed with dividers. The result is either a flat battery (a cheap cell in a silver casing), or a prismatic battery (a fashionable battery that you will not find on a laptop), or if you twist it all into a tube - a cylindrical battery (for example, 18650, or AA) .
Million Mile Battery
You may have already read the news that Tesla promises to release a battery that lasts a million miles. The real work was carried out by a group of researchers from the University of Dalhousie in Halifax (Canada) under a contract with Tesla, however, they conducted a lot of tests on various Li-ion batteries to find the best combination of chemical elements, as well as usage and charging profiles. A Million Mile Battery is just a marketing entrapment describing studies that optimize the chemical formulas of batteries to increase their battery life. The work itself is filled with technical jargon , so I studied the issue of batteries all weekend to make a selection.
The first thing worth noting about their “one million miles” formula - it is not typical for the behavior of most of today's drivers, average car owners who drive to work and home. Scientists have aimed at such a use of the car, which involves constant travel and battery charging after an almost complete discharge. This situation is suitable for trucks, taxis and buses. They use the term 100% DOD, i.e. “Discharge depth” is when the battery is used up to the stop, and only then it is charged, unlike, for example, a smartphone that is charged every night, regardless of the state of the battery.
What came to light: batteries like cold; hot new formulas
They found that temperature is very important. A battery that has worked most of its life at 20 ºC will last longer than one that worked at 40 ºC; however, batteries operating at high temperatures, and then falling into low conditions, lose capacity at the same speed as batteries that always operate at low temperatures. In other words, at high temperature the battery loses capacity faster, and at low temperature it loses it not so fast, and any battery can move to and fro on this graph without any memory effect. Lower temperatures provide a lower rate of degradation at the molecular level - fewer cracks, dendrites, gas pockets, etc. They very much rested on how important it is to keep everything at a low temperature.
In previous experiments, researchers spent a lot of time studying other combinations of chemical elements, but settled on the NMC532 graphite electrodes (like most of the scientific community). In chemistry, NMC532 is another name for LiNi 0.5 Mn 0.3 Co 0.2 O 2 . In simple terms, this means that the cathode consists mainly of lithium crystals, with the addition of a small amount of nickel, manganese, cobalt and oxygen, and the anode consists of graphite (although studies of graphene look promising).
However, the NMC532 / graphite characteristic is not exhaustive for the battery. It is also necessary to indicate the electrolyte. An electrolyte is a mixture of LiPF 6 , solvents and additives, with names too ridiculous to be spoken aloud - such as dimethyl carbonate or ethylene sulfate. In this work, they tested several combinations of solvents. Additives can also affect cell performance by increasing the charge / discharge rate by shortening the life time, or vice versa. Based on previous studies, they really liked the two supplement formulas (2% FEC + 1% LFO, and 2% VC + 1% DTD), although they found that they behaved differently at different temperatures, and suggested choosing the additives according to the intended application. In the manufacture of batteries, dry components are usually made first, to which liquid electrolyte is then added (Sparkfun did a detailed article describing the production process).
By choosing a special formula and maintaining a low operating temperature, the researchers were able to minimize the two main causes of battery degradation; loss of lithium and increased impedance. On average, over time, lithium ions, moving here and there, climb into places that do not allow them to work. They can turn out to be electrically isolated, grouped into plates, dendrites and surface films, react with other components of the cell, and not participate in charging and discharging. Dendrites are especially harmful - these crystals in the form of sharp lithium needles can pierce the separators and short-circuit the cell, which then heats up and leads to a self-sustaining reaction, and eventually to an explosion. Impedance increases due to corrosion of the electrodes and loss of usable surface area, due to chemical reactions, cracking, or the formation of a resistive surface layer that blocks the electrode.
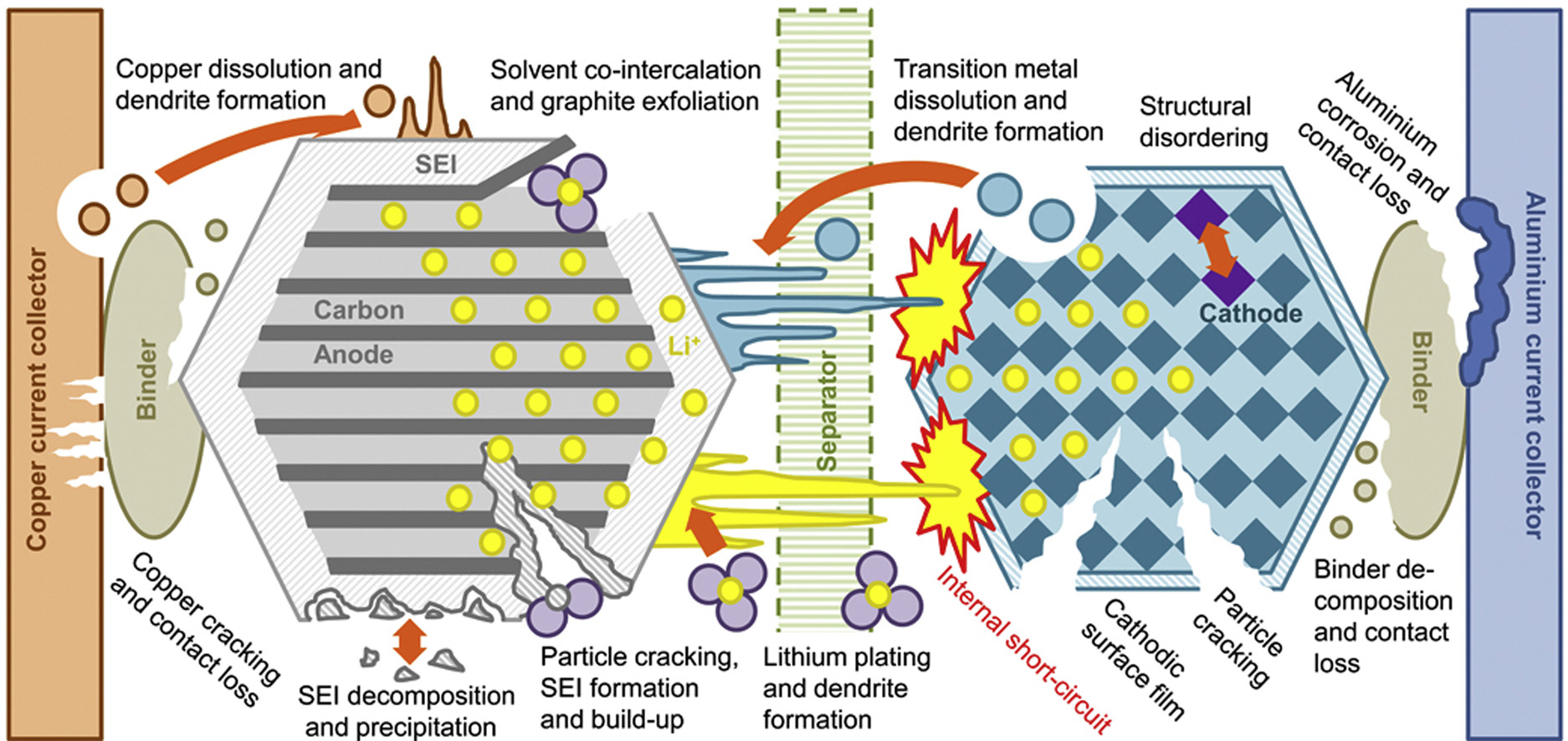
Methods of battery degradation. There are a lot of them, but in essence they come down to the fact that "atoms move where they don’t need to"
One of the reasons that their study attracted increased attention was that it was scrupulous and open. It took three years, it took him to run each battery through thousands of charging and discharging cycles using extremely accurate charging and discharging devices that record the battery capacity - and all in order to get the most complete data. It is usually quite difficult to measure the battery life cycle in accelerated mode; Batteries are subject to higher charging / discharging speeds than is required in normal life and receive less recovery time. The fact that the researchers spent so much time on their work suggests more realistic results. They also explicitly indicated:
In contrast to reports describing the use of commercial cells, we included a full description of all the details of our batteries, including the composition of the electrodes, the composition of the electrolyte, the additives used, etc. This is done so that other people can reproduce these cells and use them for their own checks.
It’s nice for a change to see a study with commercial support, posted in the public domain, and even under a Creative Commons license.
Despite the openness of the work, we probably will not see home-made Li-ion batteries in the near future. Perhaps we will see a gradual transition to the proposed formulas, and we would like more influence to be given to cooling, since this significantly increases the battery life. We are sure that if you need a battery, Tesla will soon be able to sell it to you from one of its gigafactories.