
Problem
Mitochondria generate energy in every human cell. Mutations in mitochondrial DNA, inherited or acquired during life, lead to metabolic, neurodegenerative and cardiovascular manifestations of aging.
The SENS Research Foundation has developed seven practical strategies to “repair” the major aging factors. While some of these strategies are being widely studied by the scientific community, the MitoSENS mitochondrial damage control strategy is one of the newest. Our theory is that using "allotopic expression", that is, by placing functional copies of critical mitochondrial DNA (mtDNA) genes in the cell nucleus, defects resulting from mutations in mtDNA can be eliminated.
When proposed, this unique and ambitious strategy was perhaps too "bold" for many laboratories and funding organizations. Thus, MitoSENS was an “internal” SENS project that would not have been possible without community support. So far, this community-funded approach has a great history leading to revolutionary discoveries.
In 2013, SENS launched its first crowdfunding campaign specifically for MitoSENS in partnership with LongeCity . This small initiative aroused considerable interest and paved the way for a larger fundraiser in 2015 on Lifespan.io . Breakthrough discoveries followed, and for the first time in human cells, scientists showed the fundamental work of the MitoSENS approach .

To approximate its clinical application, SRF soon created a " highly variable mouse model ." This mouse has a unique modification in their nuclear genome that allows targeted insertion of new genes at a specific location. Using this mouse, we are ready to take the next step and continue mitochondrial gene therapy in the animal model.
Decision
C57 / BL6MT-FVB line mice (let's call them “ Sick Mice ”) have a genetic defect (mutation in the mitochondrial ATP8 gene) and exhibit several age-related symptoms, including decreased fertility, arthritis, type II diabetes mellitus and neurological disorders. Since mitochondrial DNA is inherited only through the mother, crossing the female Sick Mouse with male mice from other lines will lead to the same mitochondrial dysfunction.
We will use the most variable mouse model to design a new transgenic mouse (“allotop ATP8 transgenic mouse - MitoMouse ”). This mouse will have the ATP8 gene, important for mitochondrial function, “hidden” in the cell nucleus and, therefore, able to be transmitted to offspring regardless of gender.
Our hypothesis is that both males and females in the offspring of the Sick Mouse and MitoMice will show restored mitochondrial functions. This would mean the viability of the MitoSENS strategy, showing that functional backups of mitochondrial DNA genes in the nucleus can replace their mutated analogues in live animals.
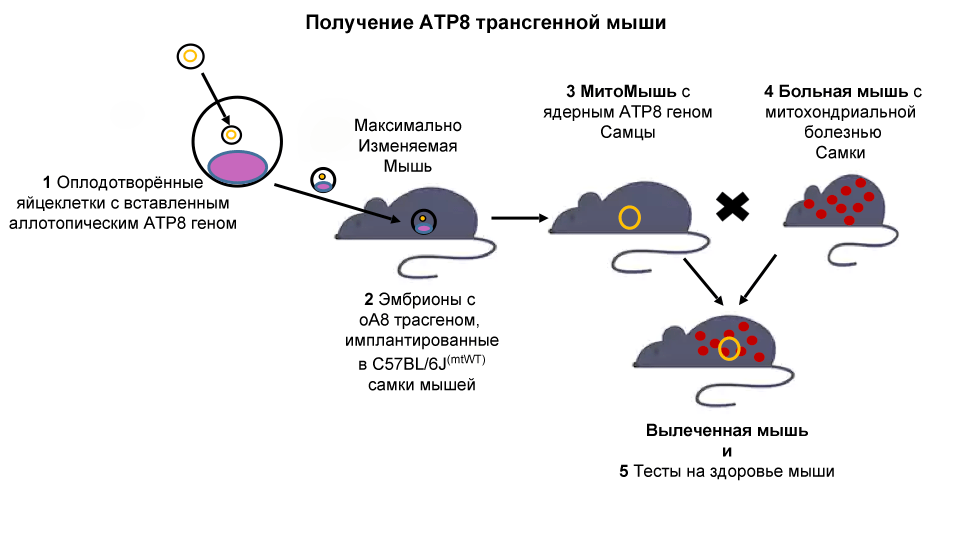
Finally, mice will be evaluated for phenotypic improvements, such as behavioral, physiological, and biochemical changes. The success of the project will lay the foundation for successful gene therapy in humans.