Clinical researches. How to get into the experimental group, get free cancer treatment and help science
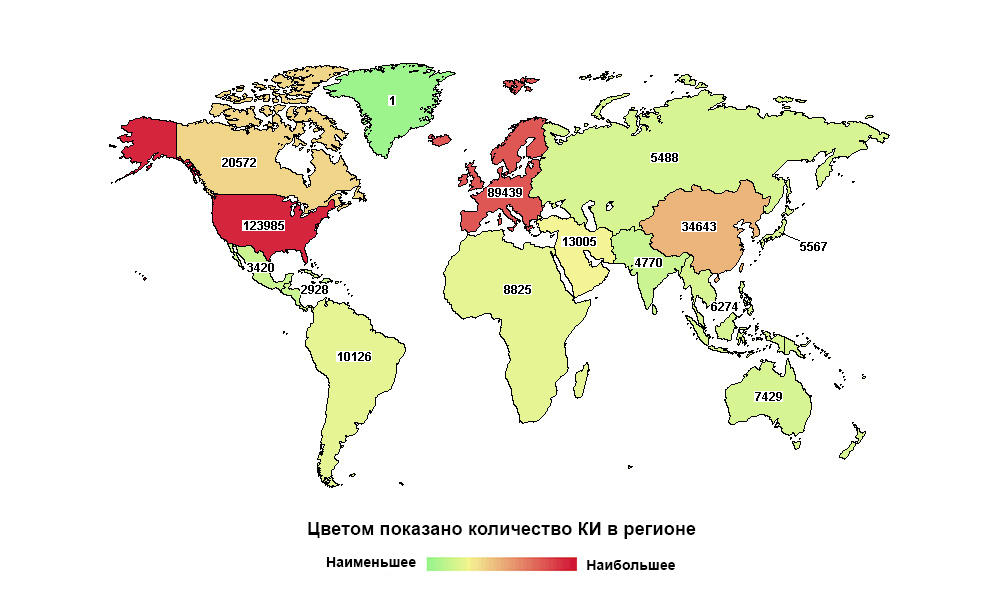
Russia is far from the first in the world, but the first in the number of studies in its macroregion
Any medicine today, before getting to the patient, goes through a long series of clinical trials. It is necessary to prove that it is able to solve a specific health problem, and to do this more effectively and, preferably, safer than its predecessors.
The selection is tight - 98% of all the studied drugs do not reach patients. At 2% of the “lucky ones”, scientific research on a new substance before entering the market takes more than 12 years and more than 1.5 billion dollars.
We at the Medicine 24/7 Clinic are directly involved in clinical trials. For the second year in a row, we have been conducting clinical trials of foreign antitumor drugs. With our help, new medicines get access to Russia faster, and more than 100 people a year - another chance for treatment, for free.
For private clinics, the practice is unusual: a minimum of commercial benefits, too many difficulties in organizing the process and strict requirements for a medical institution. Usually only large federal centers manage to meet them.
But for many patients in Russia, a clinical trial of the drug is the only chance to receive free treatment for a deadly disease. But among Russian cancer patients, 30% simply do not know what a clinical trial is, and only a few took part in them.
Therefore, we want as many people as possible to learn and check: perhaps they have a chance to get a drug that can save their life.
In this article we will explain why we need and how clinical trials are organized, who and how can get there.
Sad stories. Why clinical trials are needed and why without them - bad
Clinical research / trial (hereinafter - CI) - a scientific study involving people as subjects, which is carried out in order to assess the effectiveness and safety of a new drug or expand the indications for the use of already known. In addition to drugs, CIs can also study the efficacy and safety of new treatments and diagnostics.
Medicine is evolving and turning into an exact science, which cannot do without statistics.
Previously, the family doctor knew the stories of all his patients by heart, the doctor could live all his life in one town, find and remember a personal approach to treating everyone. Moreover, the choice of potions was small: healing herbs, leeches, mercury and arsenic. Responsibility during the postulate of "all the will of God" on the doctors was less.

Arsenic from the end of XVIII "restored" potency and "cured" arthritis ...

... and mercury, for example, was a laxative and "from syphilis."
When medicine became widespread, doctors needed to develop truly unmistakable treatment tactics. Certain drugs were supposed to help most patients under given conditions.
Ideally, a physician should use only those methods of prevention, diagnosis and treatment that have an extremely low probability of obtaining “random results”, because their usefulness and effectiveness have been proved by many correctly conducted experiments.
This is evidence-based medicine - the only adequate approach to such a serious matter as human health today.
And it is clinical research that is the basis of evidence-based medicine.
Until the middle of the 20th (!) Century, there was no regulation of research on new drugs. To restore order, as often happens, it took a couple of tragedies.
In 1937, 105 children and one adult died, taking the "elixir" from the antiseptic sulfonamide and ... poisonous diethylene glycol. Yes, the one that is used today in antifreeze. Then the pharmaceutical company ME Massengill unknowingly used it as a solvent, excipient. No safety studies of the resulting “cocktail” for humans have been conducted. When they suddenly realized and seized the drug from sale, there were already more than a hundred victims. In 1938, the U.S. Congress passed a law on mandatory drug research before it goes on sale. Control over this was entrusted to the FDA ( English Food and Drug Administration), the Food and Drug Administration
An even louder scandal occurred with thalidomide in the late 1950s - early 1960s. "Soothing and sleeping pills, which perfectly helps with toxicosis of pregnant women" sold out quickly. His studies were carried out only on rats. It turned out that this is not enough. In humans, thalidomide caused defects in the development of the fetus. In Europe, Australia and Japan, about 10,000 children were born with malformations (malformations) of the limbs. The drug was banned in most countries in 1961.

Moms of these children drank sleeping pills, not tested in humans
Since then, medicines have been carefully studied before registration. This is regulated by the International Harmonized Tripartite Rules of Good Clinical Practice (ICH Harmonized Tripartite Guideline for Good Clinical Practice, abbreviated as ICH GCP). From 1996-97, they operate in the United States, Japan and the EU, and since 2003 they have been introduced in Russia.
How is the study going and why for so long?
The whole process of creating the drug can be divided into 3 large phases.
1. Search for ideas and preclinical studies - in vitro and in animals.
2. If this did not end there, then clinical studies begin, with people: first careful, then more massive.
3. The drug is then registered with regulatory authorities to become a familiar name in medical directories.
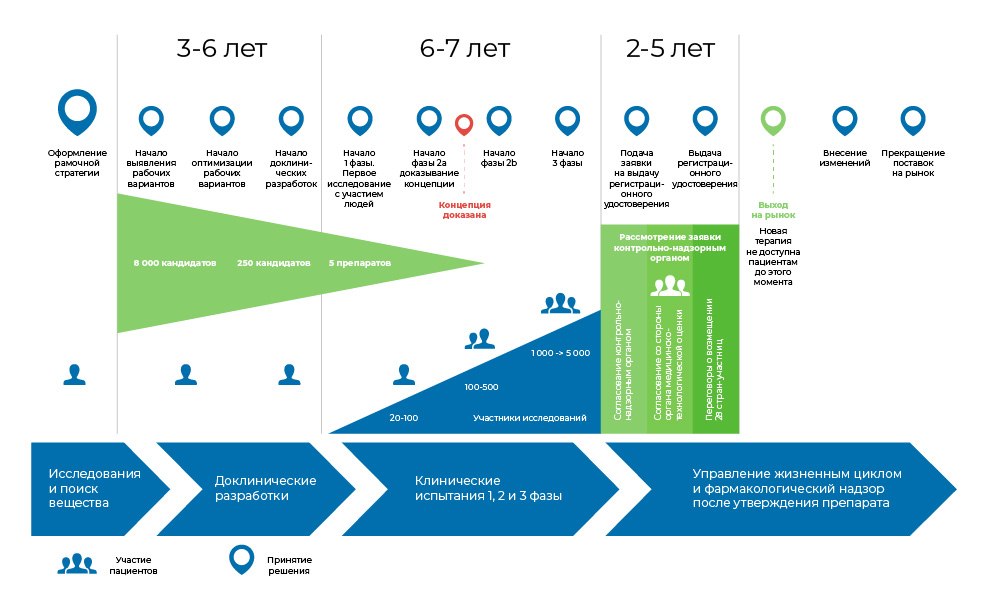
The process of developing a drug. From the moment the molecule is created until the start of the sale of the drug, it takes from 8 to 20 years.
So does anyone need this? Oncology is one of the most egregious areas of medicine in terms of the unmet need for drugs. According to the World Health Organization, in 2018, cancer killed 9.6 million people. Tumors are often found in the later stages, when only palliative treatment remains.
At the same time, discoveries in the field of molecular biology and genetics made it possible to understand the mechanisms that contribute to the development and progression of cancer, and the understanding of the work of antitumor immunity improved.
And today, the development of anticancer drugs is one of the most science-intensive and popular areas of medicine.
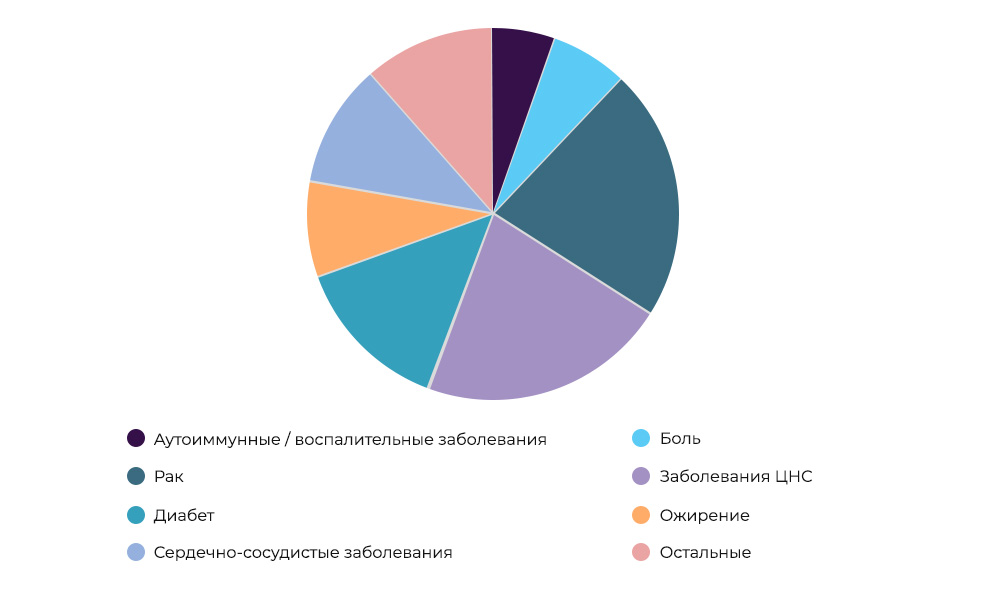
Research on anti-cancer drugs - 23% of all CI in the world
Who pays for the research. Sometimes the organizer and sponsor may be a research organization. But more often, scientists are engaged in scientific research at the expense of pharmaceutical companies. Those expect to enter the market with a successful drug, make a profit and recoup the costs of CI and development. It’s like buying a new movie for rental in a movie: the distributor does not know whether he will “shoot” or not. Producing new drugs is a very risky business.
Previously, many pharmaceutical companies conducted research on their own, using the staff of scientists. Now a medical institution that has passed accreditation and meets certain requirements can become a platform and performer for the experiment.
This is exactly the case of our “Medicine 24/7” . The pharmaceutical company is ready to pay, but finances are allocated at the end of the study, on the fact of the costs incurred. The clinic does not earn any superprofits. And research doctors do not earn anything from this on top of their usual salary. Rather, this is the position of the head of the clinic: a person considers it right to move the country's medicine forward and takes the opportunity to participate in this.
First an idea comes up. Actually, what to investigate? In oncology, “targets” are first found - a weak spot of the disease. If you disrupt or simply "turn off" the work of target molecules, the tumor "suffers". This principle is based on many modern cancer drugs - targeted or immunotherapeutic .

The mechanism of targeted drugs in colorectal cancer. Cancer cells stop dividing, or grow additional blood vessels to the tumor, or the drug protects neighboring cells from becoming malignant
To find such substances, and then choose the most suitable candidates, it takes a lot of resources and time for in vitro and in silicio studies - that is, in vitro or using computer simulation.
The selected substance is stored in the right amount - produced according to special rules (in Russia it is GOST R 52249-2009), without impurities and violation of technology. And with these test tubes, scientists go to test the drug on animals.
The mouse is the engine of progress. After testing ideas in vitro, a scientist with a supply of his potential drug goes to the vivarium - you need to check how the prototype behaves in the body of a mammal (in vivo).
Even Avicenna in 1025 in the "Canon of Medical Science" wrote that medicines must be checked. Moreover, it is desirable - on a potential patient, a person. After all, the result obtained on lions and horses does not guarantee that the medicine will affect people the same way.
And still in medicine, without experiments on animals - can not do. The lions and horses, however, were left alone. Preclinical studies around the world occur mainly in mice, guinea pigs and rabbits.

Laboratory mice even put a monument in the Novosibirsk Academgorodok
At this stage, check how harmful / safe the drug is:
- does it cause allergies
- does it have a toxic effect on tissues and organs,
- how it affects the ability of animals to reproduce and normal development of the fetus, etc.
In addition, they observe how the drug candidate behaves inside the body of the animal (pharmacokinetics):
- absorption rate and increase in blood concentration,
- what are the maximum and minimum dose
- how quickly it is excreted from the body, etc.
All these data are needed to decide whether it is possible to use the test substance for humans. And if so, how much is needed.
Inevitable evil. Bureaucracy. The Department of State monitors the correct progress of CI . regulation of drug circulation by the Ministry of Health and the Federal Service for Supervision of Healthcare (Roszdravnadzor).
If the scientist has come to the moment when it is necessary to proceed to clinical trials in humans - it is time to prepare an application for conducting CI. To do this, he needs several documents .
- Dossier of the study drug. Everything that has already been found out about the drug: data on pharmacokinetics, effectiveness, toxicity, etc.
- Study Protocol It describes in detail the plan for future research and methods for assessing results;
- Researcher Brochure. A short cheat sheet to clearly explain the essence of the study to volunteers and patients and get their informed consent.
Ethics Committee. The next stage of the quest is to get the assessment and conclusion of the ethics committee.
The Ethics Committee is an independent group of doctors, scientists, medical personnel and non-specialists (members of the public). They study the research protocol and informed consent to make sure that there is agreement between the patient, researchers, the pharmaceutical company and the national regulatory authority, that no rights are violated, that no one is subjected to coercion, and that no one infringes on free will.
After severe side effects from one drug in 2006, the ethics committee became even stricter. Therefore, sometimes the study may “freeze” at this stage for a year or more.
After checking all the documents and approval of the ethics committee, the potential drug goes into the stage of clinical trials - in humans.
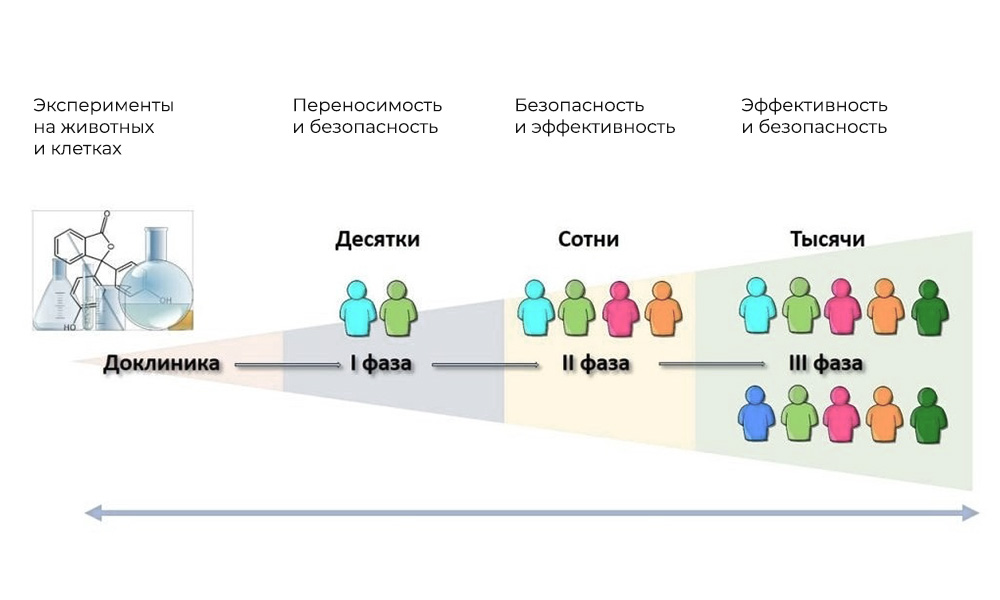
The main phases of clinical trials are in humans.
Phase I. Testing the mechanism of action
Participants: 20 - 100 people.
Duration: from several months to 1 year.
Objective: to study tolerance, pharmacodynamics and pharmacokinetics.
It is checked whether the substance acts on humans in the same way as on animals, whether it is safe.
In the first phase of a clinical study, theoretically, healthy volunteers should participate, but in oncology testing of potent substances in a healthy body cannot be called ethical. Therefore, people with the corresponding disease are involved, against which the future drug may be effective.
Participants are gradually injected with all larger doses of the drug, starting from the minimum to the maximum allowable. After each administration, the patient's condition is monitored.
Pharmacokinetics are evaluated : absorption rate and excretion (excretion of unchanged substance), distribution over tissues and organs. Pharmacodynamics are also evaluated: the effect of the drug on tumor cells, on other takni and organs, side effects. The preferred application and dosage level are being clarified.
In addition to studies with increasing doses, in phase I check:
- the effect of food on the drug;
- interaction with other drugs;
- the effect of other diseases that may affect the desired dose of the drug (for example, in a patient with renal failure).
According to the FDA , 70% of drugs successfully pass the first phase of CI.
Phase II Checking the action for a given goal: a specific type of disease
Participants: 100 to 500 patients.
Duration: from several months to 2 years.
Purpose: to test the effectiveness of certain indications
It is necessary to examine how effective the new drug is compared to placebo or existing treatment. Plus, a greater number of participants can detect rarer side effects that are not detected in phase I.
To participate in this phase of CI, patients are selected according to a much larger number of criteria than in the first phase. For example, not just “breast cancer,” but “breast cancer, stage T2N1M0, HER2-positive subtype”.
Typically, studies at this stage are conducted as double-blind, randomized, placebo-controlled.
Double blinding: neither the doctor nor the patient knows who is receiving the active substance and who is receiving a placebo or the optimal treatment currently existing.
Randomization implies that patients are divided into groups randomly - using a random number generator. Neither the doctor nor the participant in CI can influence this process.
Placebo control means that participants in one group will receive a placebo under the same conditions as participants in another group who are given the active substance.

Everyone - the same in appearance, taste and smell of the medicine.
All this "conspiracy theories" is needed to exclude intentional or unconscious distortion of experimental data by participants or researchers.
So, in the first phase, where there are no such strict requirements, there are stunning results. This is a “dirty” statistic; in phase II it is cleared of excess and the results become plausible.
According to the FDA, only 33% of drugs that reach phase II successfully undergo CI and move on to the next phase.
Phase III Supporting studies
Number of participants: 300 - 3,000 or more.
Duration: from a year to several years.
Purpose: confirmation of the effectiveness and safety of the test substance in large samples.
This is the largest, most complex, and most expensive part of the drug development process. The purpose of such studies is to confirm the effectiveness and safety of the test substance when used by a large number of patients.
According to the results of this phase, the manufacturers of the drug receive permission to bring it to the market.
In phase III, thousands of patients from different countries can take part. Everything should be planned to the smallest detail, so that in all places of the study its design and significant conditions are exactly the same.
The design of the study is so narrow that not only a dying patient, but also a patient with a prognosis for stable remission can get into it. The drug should be so safe that it can be given to a healthy person - and the quality of life does not decrease.
Before the start of phase III, there are a lot of consultations and discussions between researchers and third-party experts: it is very important to think over the design of experiments so as not to miss the important and get all the necessary data.
During phase III, the efficacy and safety of the new drug and the dose-dependent effect are finally confirmed.
The correlation of advantages and risks is analyzed. Based on the results, the regulatory body decides whether it is possible to bring the drug to the market. To do this, the following conditions must be met:
- the drug is more effective than previously known analogues,
- gives less side effects / better tolerated,
- effective when previously known drugs do not work,
- more profitable economically,
- easier to use.
The review process of the application by the supervisor takes 12-18 months.
According to the FDA, the third phase of clinical trials ends with a positive result in only 25-30% of cases from all that were at the start of the third phase.
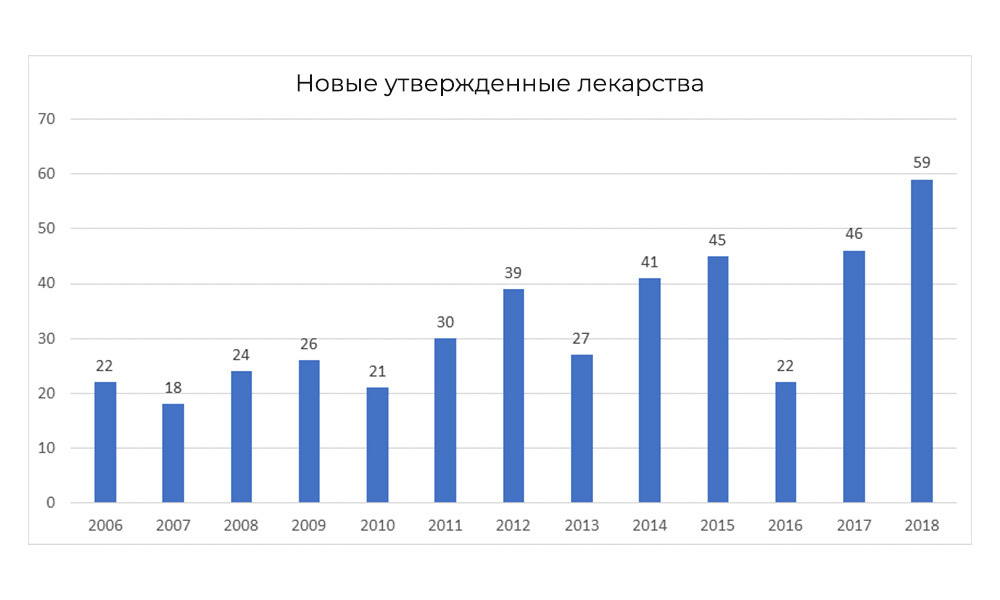
However, in 2018, the FDA broke its own record for the number of approved drugs.
Features of national research: an additional phase of CI in Russia
The control over new drugs in Russia has its own bugs (or features, how to look). By law, approved foreign drugs must undergo additional clinical trials in Russia: supposedly, this will increase the quality of foreign drugs.
When the drug has already passed 3 phases, went on sale to the world market, in our country it may still not be registered. We know that it works, in its instructions in foreign languages there are indications confirmed by 3 phases of research in the USA, or Canada, or Europe.
Let's say 12 indications - 12 diagnoses in which the drug will be effective. But in Russia, not all of these indications have yet been officially confirmed, only 6 out of 12. And if the patient has exactly the type of cancer for the treatment of which the drug has not yet been officially registered in the Russian Federation, he will not receive such a drug for free, as part of the compulsory medical insurance.
This slows patients access to new drugs for 2-3 years.
24/7 Medicine is involved in Phase III research.
This is how we contribute to speeding up the drug registration process. The faster the pharmaceutical companies have data on the required number of patients, the faster the medicine will be adopted. First all over the world, then with us. As a result, we influence that everyone can receive it - not only in private clinics, but also in any state oncology dispensary, according to compulsory medical insurance.
In addition, for our patients, participation in phase III clinical trials is an opportunity to receive a sufficiently proven and safe new treatment - without several years of waiting until it officially “reaches” Russia.
Unfortunately, we are few in number. Not every clinic can receive CI.
First, the clinic must have a GCP certificate , Good Clinical Practice.
Secondly, there should be at least two researchers. They also have certificates, and it is not so easy to obtain them: they are issued by the regulatory body of the country of manufacture of the drug. In our case, these are the USA and France. Two doctors were checked for six months.
Thirdly, the clinic itself. It is mandatory to have a resuscitation department, its own laboratory, certified nurses, the correct calibration of the necessary machines, certain rooms for storing drugs and documents, anonymous rooms where there are no cameras - for patients. To start for the first time, I had to re-equip some rooms and wards: up to new beds, all according to the design of KI.

For different drugs - different refrigerators
In a word, those clinics and doctors who want to do this should still try to create certain conditions.
How is the study
The substance itself and a thick folder of 1,200 sheets are brought to us in an atmosphere of complete secrecy, where the design and protocol of the study are prescribed. Every trifle is indicated there: how, at what time and in what doses we should administer the drug, how often and how much to take blood for analysis, what tests to conduct, how often to do MRI and CT. How many times a day and in what form to send “encryption to the center” - reports to the organizing company KI.
We recruit 2 or 3 groups of patients, sign an informed consent with them.
Usually people agree to participate in clinical trials for 2 reasons:
- lack of effective treatments for their disease,
- desire to contribute to the development of science.
In any case, this is an exclusively voluntary decision. Researchers are required to provide the patient with information on the purpose of the CI, how it will be administered, what medicine will be used, etc. All this is reflected in a special document - informed consent . Each study participant is insured against harm to life and health.
Our patients have never refused yet - for them this is an incredible chance to receive treatment, and free of charge, when other methods of therapy have already exhausted themselves.
Of course, taking everyone into the study will not work. Requirements are given "from above", and this is a very narrow set of parameters: how old the participants should be, what exact diagnosis, there were / were no operations, the level of creatinine and iron in the blood is before the decimal place.
But we have a lot of cancer patients. If the client admits that there is no money for treatment, we will definitely check the requirements for the study participants. It is possible that he will find a place.
A set of 18 large-scale studies is currently open.
Of course, the scale in a private clinic is not at all that of a large research institute, but usually 3-4 patients a week fall into the study protocol. This is a lot: at least 10 people a month who receive the most advanced advanced treatment - for free.
And they themselves have an additional chance, and future patients are more likely to be able to get this drug when the end of CI.
Patients do not necessarily have to be constantly in the clinic - if it is not prohibited by the design of CI and the patient feels good - he participates on an outpatient basis. Comes, gets his dropper and leaves. There are those who are better off staying in the clinic under supervision.
As a result of long months of scrupulous fulfillment of a long list of requirements, constant monitoring of the patient’s condition, consolidation of data on dozens of parameters, we are doing research that will be published, and all the doctors from all cancer centers of the country will use the results - the expanded ability to use new drugs.And dozens of people get a chance for treatment right now.
We hope this text was just an interesting read for you.
But if the topic is close to you, take a look at the lists of clinical trials on RosOncoWeb , CTAgency and on the website of the Ministry of Health . And be sure to contact the oncologists of “Medicine 24/7” - you need to take a chance.
All Articles