Personal cancer cure. How Patient Genes Affect Treatment Success

The successes of modern clinical oncology are undeniable. More and more complex operations, new drugs, effective methods of pain relief and elimination of painful symptoms. We in our blog talked enough about how today it is possible to extend and make life easier for patients even in the last stages of the disease.
But, nevertheless, thousands of cancer patients all over the world daily learn that a tumor that yesterday gave in to certain treatment is growing again today or metastasizing. Doctors regularly find themselves at a standstill: all the prescribed medicines and treatment methods have been tried, and there are no effective drugs left for this patient.
However, even a way out of this impasse can be found. With the development of genetics and molecular biology, oncologists have found a new way to study the tumor in order to find vulnerabilities in it.
To do this, use molecular genetic testing - determining the characteristics of the DNA of cancer cells. The method is technically complicated, expensive, it requires specific knowledge from a doctor.
The study takes 3 weeks, costs from 250 to 670 tr As a result, the doctor receives a report of 30 pages of complex information, which he should still be able to use. But for patients who have already ceased to hope, this gives additional life time.
At Medicine 24/7, we regularly resort to molecular genetic research to treat a person when "everyone has tried it - nothing else to do." And the patients, who seemed to be nothing more to help, continue to live. Some are two months instead of two weeks, and others are years instead of a couple of months.
Today we want to talk about how molecular genetic testing is done, in what cases it can help the patient and what knowledge the doctor gives.
We are all mutants, this is the norm. But some mutations lead to cancer
“Decent” cells live without disturbing others. They use a strictly allocated amount of resources, adequately perform their biological functions, and in due time they die, giving way to next generations (this process is called apoptosis). Every 7-10 years, the human body is completely updated.
For this, all somatic cells (those that make up the body), except for red blood cells, continuously divide.
Before dividing, the cell is stocked up with a “copy” of hereditary genetic information that is in its core. DNA strands “folded” into chromosomes inside the nucleus are replicated, i.e. double up. And after this, the cell divides, quietly distributing to each of the daughter cells an identical set of chromosomes. From one cell, two are absolutely the same, and together with their genetic baggage each of them gets “knowledge” about how it needs to live, what function to perform, and how many times in life to share.
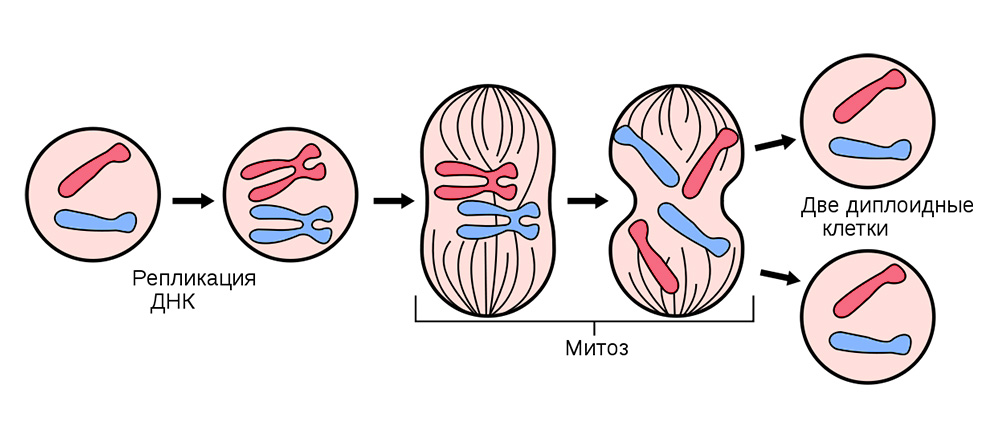
Somatic cell division occurs in all organs and tissues.
Sometimes in the process of division failures - mutations turn out. Either the DNA strand will break, then it will be copied with an error, then the chromosome sections will mix. Hundreds of factors can affect this: from stress and tobacco smoke to exposure to radiation.
Mutations can be divided into 4 types.
1. Replacement of a base pair (Single-nucleotide polymorphism, SNP): one nucleotide - the "letter" of the genetic code - changes to another. The structure of the protein encoded by this nucleotide sequence is also disrupted.
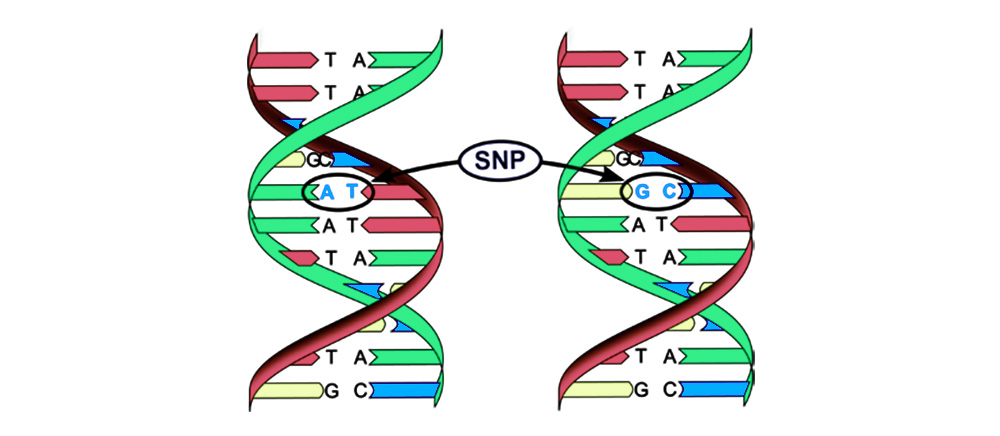
2. Chromosomal aberrations.
Deletion - loss of a chromosome site. They occur due to the termination of the terminal portion or DNA rupture in two places at once. That's it - this gene is no longer expressed on the chromosome.
The torn off "pieces" of DNA can be inserted into the adjacent chromosome - an insertion (or inversion if insertion occurs in the reverse order) will result . Sometimes between the chromosomes there is a "mutual exchange" of DNA sections - translocation . The result is one: "extra" genes are expressed .
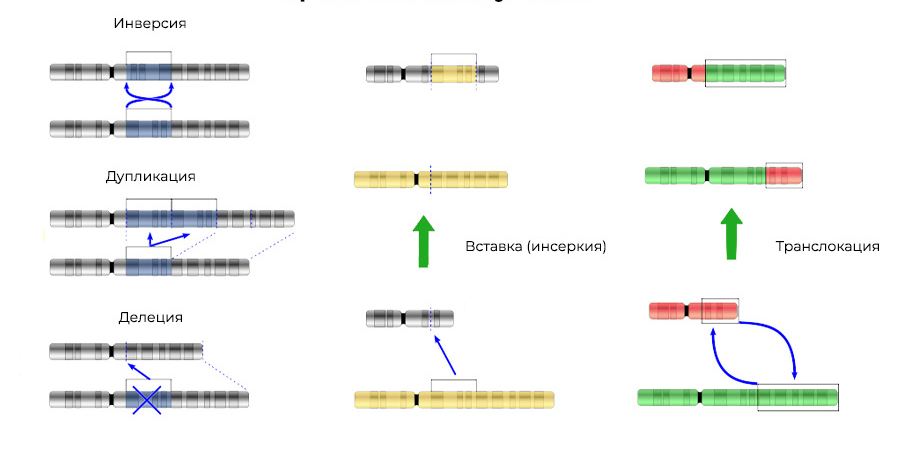
Mutations change not only the structure of a DNA site, but also the order of these sites
3. Gene fusion - a gene is "assembled" from parts of other genes and expressed (triggered) as a whole. The protein during the expression of such a chimeric gene also turns out to be unusual, hybrid, with harmful properties.
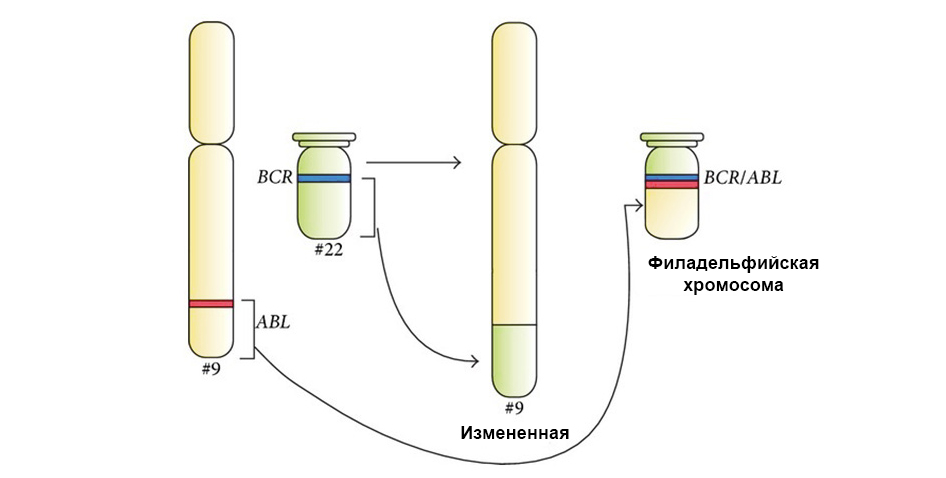
Two genes “stuck together” during translocation and formed a chimeric gene (causes leukemia)
We are lucky that the DNA is full of not too significant sections that do not encode anything. Many mutations occur in these areas - and turn out to be insignificant, have no effect on the further work of cells. And such a cell with a slight difference from the “standard” continues to live and share normally.
Over 70 years, 100 trillion cell divisions occur in the human body. This is 1.4 trillion divisions per year - there are enough cases to accumulate a “critical mass” of errors in DNA over time, or so that another failure could still get into the DNA section encoding something important. This will result in a not-harmless mutation, due to which the cell will become malignant (malignant).
A malignant cell is distinguished from a normal cell cycle disorder.
The cell cycle (cell life from division to division / death) is strictly regulated by the work of special proteins: kinases, cyclins, growth factors and transcription factors - there are dozens of them in every living cell, and each has its own special, but important function.
They transmit signals between the cells of a multicellular organism, activate each other, start the process of division and control its correctness, maintain the correct activation of genes, perform their functions by the cell, “check” the genome integrity, “command” the cell to start apoptosis if it is time to die, and t .P.
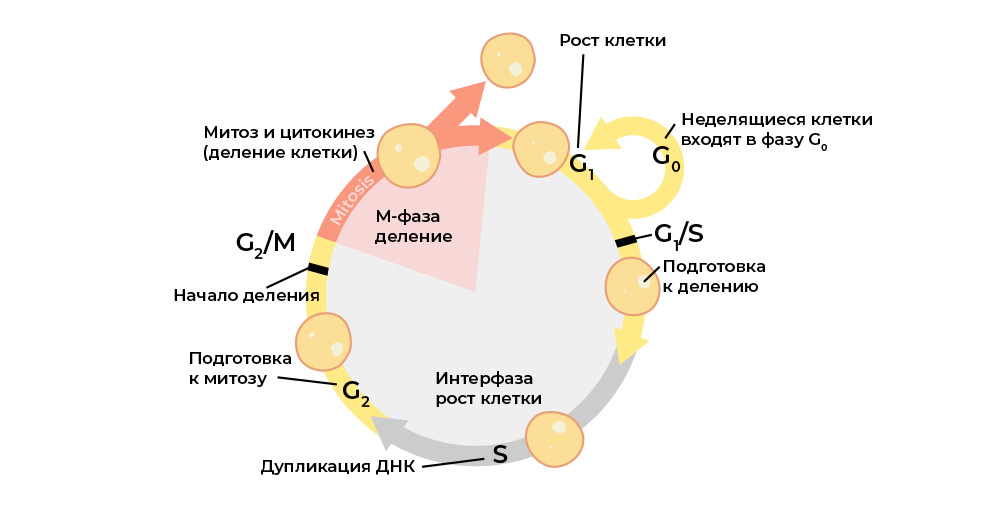
Each stage of the cell cycle is controlled by regulatory proteins.
And each of these proteins is encoded in a specific part of the DNA - the gene. If such a gene undergoes a harmful mutation, it will “reproduce” the corresponding regulatory protein incorrectly. And the “wrong” protein-regulator will disrupt the cell cycle, and with it the behavior of the whole cell.
For example, the gene for the protein regulator of proliferation (cell mass growth) “breaks down” - and the “mutants” begin to divide more than they should, healthy cells “crush”.
There are two large groups of such significant genes, changes in which can lead to carcinogenesis (the onset of cancer).
Protooncogenes are “normal” genes that can become oncogenes due to the enhancement or change in their functions. Genes whose expression can lead to malignancy of the cell and the development of neoplasms are called oncogenes . If a harmful mutation occurs in the proto-oncogen, it becomes an oncogen and can cause a tumor.
Of those that are most well-studied and are widely heard:
- EGFR, ALK, BRAF - non-small cell lung cancer;
- BRAF - melanoma;
- HER2 - breast cancer (breast cancer);
- KRAS - colorectal cancer.
Moreover, mutations of these genes are found in several types of tumors. For example, increased HER2 expression is found not only in breast cancer, but also in lung and stomach cancer.
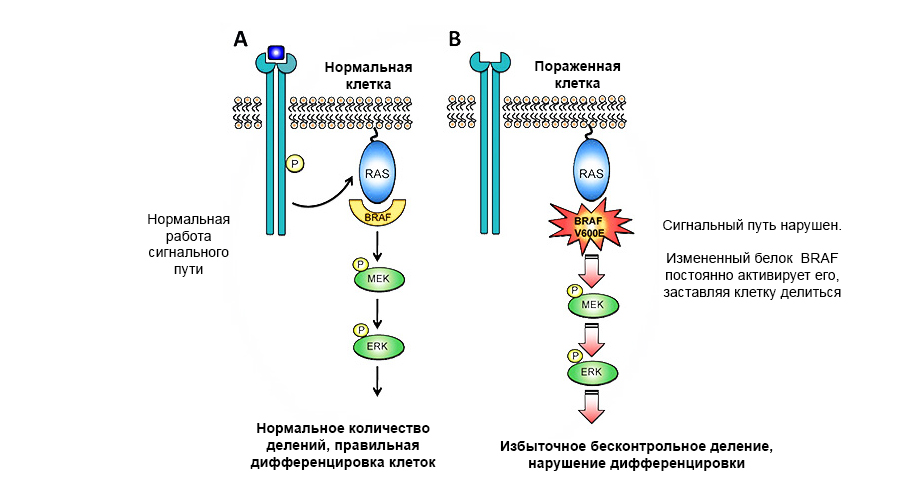
A mutation in the proto-oncogen of the BRAF protein leads to uncontrolled tumor growth.
Tumor suppressor genes (anti-oncogenes), on the contrary, can suppress the growth of tumor cells or participate in the repair (repair) of damaged DNA. But the inactivation of suppressor genes as a result of mutations dramatically increases the likelihood of a malignant tumor.
For example:
- mutations BRCA1, BRCA2 - cancer of the mammary glands, ovaries;
- mutations p53 - up to 50% of various types of cancerous tumors, including sarcomas;
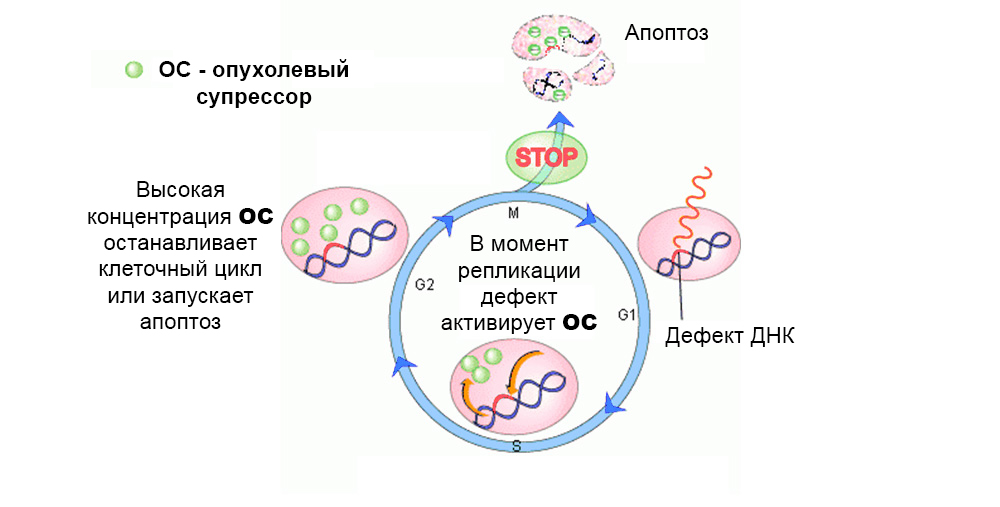
Normally, protective mechanisms act against the development of mutated cells. A defect in the tumor suppressor gene “turns off” them
In total, the effect of several tens of proto-oncogenes and tumor suppressors on carcinogenesis was studied.
Why are there so many difficulties and how do they prolong the life of patients
Each mutation found in a proto-oncogen or suppressor gene is the cause of the "superpowers" of the cancer cell, such as neglect of apoptosis and the ability to hide from immunity. But at the same time - this is its potential weak point.
Knowing what is the reason for the peculiarities of the mechanism of the tumor’s operation, one can find a substance that “wedges” this mechanism and interrupts the pathological reaction chain in the cell. That is, specific mutations in a tumor - indicate the target at which doctors “hit” the drug. This principle has allowed the development of targeted therapy .
Targeted therapy - the name of a whole branch of effective drug therapy for cancer - was born from the English word target. Targeted drugs act “precisely” on cancer cells - because only they have mutations in the corresponding genes. Healthy cells do not have such mutations - and the drugs do not act on them.
The figure below shows the mechanism of action of the targeted drug Imatinib on tumor cells with the Philadelphia chromosome mutation: the BCR-ABL fusion gene. This mutation leads to the fact that the mechanism of apoptosis ceases to work in the cell - errors in the genome accumulate, the cell degenerates into a cancerous one.
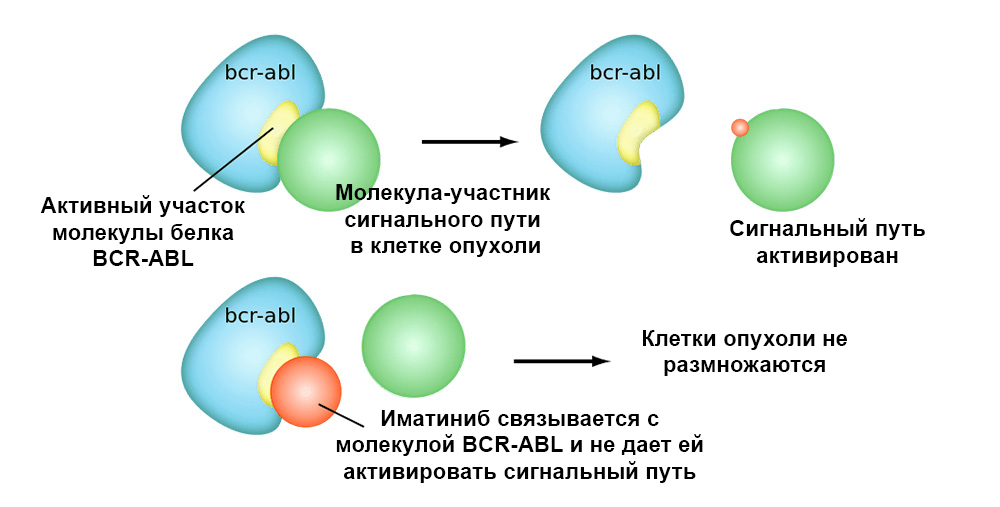
Imatinib binds to the active site of the BCR-ABL protein molecule, and blocks its ability to interact with other molecules in the signal pathways.
So targeted drugs have 2 important advantages over classical chemotherapy.
Higher efficiency. The targeted action on tumor cells allows achieving a better “response” of the tumor to treatment. For example, compared with the classical treatment, the addition of the targeted drug Trastuzumab along with chemotherapy for breast cancer with HER2 overexpression significantly increased the frequency of “responses” - 81% versus 73%, and the frequency of complete morphological remission (tumor disappearance) - 43% versus 23%
Less side effects. Classical chemotherapy with a cytotoxic effect. Essentially, toxic substances to kill or at least slow down the growth of cancer cells. Most of all they act on cells that quickly divide. That is why, for example, hair falls out of it: this is also a type of actively dividing cells, and they “fall under the distribution”. Because of this not very targeted action, chemotherapeutic drugs give serious side effects: both the gastrointestinal tract and other organs suffer.
In the practice of “Medicine 24/7” we most often prescribe targeted drugs as part of complex treatment: we combine chemotherapy, and target, and immunotherapy.
The difficulty is that each tumor is unique in its set of “targets” mutations
Just as each person has a unique DNA code, tumors are unique. After all, they are “born” from the body’s own cells. There are no tumors with the same molecular genetic properties. Therefore, creating a universal “pill” for cancer is fundamentally impossible. Cancer is too individual a disease.
But the treatment for him should be appropriate - individually selected for a particular patient - based on the fact that we determine mutations in his tumor cells.
In the recent past, malignant tumors could be classified only by histology, that is, depending on which organ they originated in and what the cancer cells looked like under a microscope.
To effectively use targeted therapy, this is not enough. The doctor should know what mutations are in the tumor cells of a particular patient, whether there are biomarkers “targets” for this or that drug. Personalized medicine as it is.
For this, we use molecular genetic studies. To find the "targets" for which you need to hit with targeted and immunotrerapevticheski drugs - you need to determine from which genes the DNA of the tumor is collected, and which genes in it are "broken". As a result:
- we learn the sensitivity of the tumor to drugs;
- find out if the tumor has resistance to certain drugs;
- discover the genetic characteristics that give hypersensitivity to drugs;
- we will choose a new treatment if the tumor has stopped responding to standard therapy;
detect a tumor / metastasis at a very early stage - by fragments of its DNA in the blood; - we can predict a favorable or aggressive course of the disease.
The sample is most often the tumor tissue, either taken during the operation to remove the primary lesion, or a biopsy - a microscopic piece of the tumor is taken with a special thin long needle.
You can search for the DNA of tumor cells in the blood - then you need a so-called liquid biopsy, two test tubes with 8.5 ml of blood each.
During a biopsy, we often encounter the fact that many patients are afraid to touch the tumor altogether - they are afraid that this will provoke it to grow. To date, no studies are available that would show such a relationship. Of course, a biopsy must be performed correctly. When we take a biopsy sample, doctors most often mark the place of entry of the needle: either they make a small tattoo (there is such a tool ), or they put a bracket (surgical). If an operation is needed later, they excise the entire course where the needle was — from the skin to the tumor — so we make the chance of cancer cells spreading beyond the tumor even less.
Next, the samples are sent to the laboratory of molecular genetic studies.
There, tumor DNA was isolated from the sample and sequenced. That is - they “read” the sequence of “letters” -nucleotides. And then they compare it with a diagnostic panel selected from the database of libraries - already decrypted genomes of thousands of other people. The panel is selected for each patient - taking into account the history and clinical data. All this, of course, is done by automatic sequencers and a computer.
And if 20 years ago the “reading” of the genome took months, it required a slow and complex decryption, today in the laboratory with which we work, an analysis is done in a few working days.
Moreover, several methods are used at once: sequencing of a new generation (NGS), Sanger sequencing and the method of fluorescence hybridization (FISH) . Together, they allow you to read the entire DNA sequence of a tumor, find out driver mutations - that is, those that launched the malignant process and can now be targeted by targeted therapy - and even visualize the entire karyotype (chromosome set).
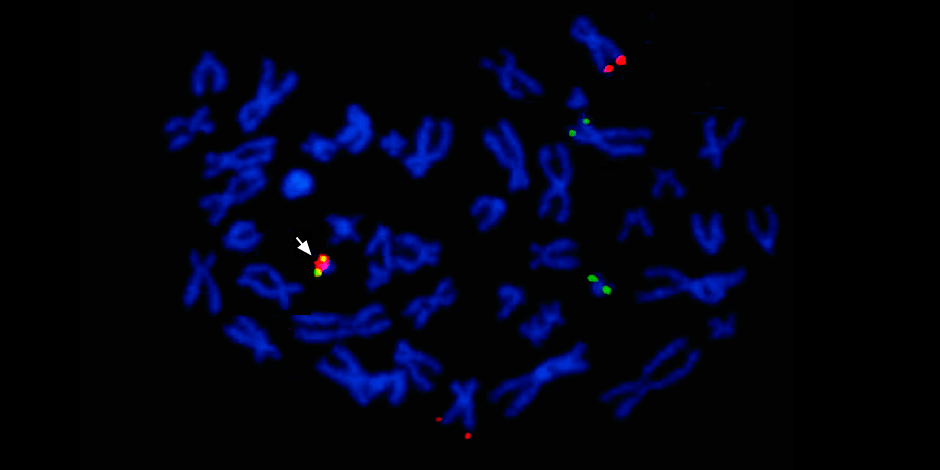
Under the arrow to the left is the fusion of the red and green signals - evidence of the fusion of the genetic material of chromosomes 9 and 22 with the formation of the chimeric Philadelphia chromosome.
In addition, a full molecular genetic study necessarily determines microsatellite instability (MSI, microsatellite instability) - a disruption in the mechanism of DNA repair, which lead to the rapid accumulation of mutations in cells. This factor allows you to make a forecast about the further course of the disease.
After obtaining the molecular genetic profile of the tumor, its analysis begins
Special programs process the results and make recommendations automatically. But then these recommendations are necessarily manually supervised by a team of experts. The analysis involves genetics, bioinformatics, oncologists, immunologists and chemotherapists. At this stage, refinements and additions necessarily occur.
Depending on the request, such a study can take from 5 to 15 working days : one patient just needs to determine the type of tumor and clarify the recommended therapy - it is enough to check the presence of a basic set of 20 DNA mutations according to the recommendations of world cancer associations. And another, with a rare diagnosis or resistance to standard treatment - you need to make a "molecular passport" of the tumor, and for this - to sequence 400 genes.
As a result, in the first part of the report, all found mutations in the patient’s tumor, and targeted drugs that will be most effective in this case are registered . Targeted therapy is approved for this type of tumor with mutations detected, and targeted therapy is approved for the treatment of other types of cancer with the same mutations. In practice, we have had cases when drugs of the second order, off-label, were prescribed and worked well.
Further, the laboratory staff does a great job of monitoring scientific research, which may be significant in the case of this patient.
The second part of the report contains an overview of studies existing at that time with detailed data on the frequency of occurrence of this mutation, on the effect of different drugs and on the possibility of using one or another type of targeted therapy for identified mutations. This helps to make at least an approximate prognosis for the patient.
The third part of the report contains relevant clinical studies in which the patient can take part in order to receive experimental treatment. This is the latest emergency method, but to know all the details about it is useful for patient peace of mind.
As a result, from this report, the doctor receives the most complete molecular genetic profile of the malignant tumor. He has information what exactly we treat, what specific breakdown in a cell. There is a “freshest” understanding of which drugs are currently approved or available for use in clinical trials.

The report is pretty weighty - 30 pages of breathtaking reading
Who needs this?
Those who have developed tumor resistance or intolerance to all drugs from the standard treatment protocol. The situation when "everyone tried it - it did not help."
In principle, the current treatment standards, especially the European and American protocols (NCCN), which we use in “24/7 Medicine”, have good therapeutic potential - it is not for nothing that they are considered the “gold standard” of treatment in oncology.
According to these standards, first-line therapy drugs are prescribed first - those that statistically best help with this diagnosis. Watching the dynamics. If the tumor does not respond to treatment, or - worse - progresses - they switch to 2nd-line drugs - because the results of the studies gave a slightly less successful treatment. If these drugs also stop helping - go to the 3rd line, etc. For many patients, the length of this “chain” is enough for the rest of their lives.
But regularly, unfortunately, doctors are at an impasse: in a situation where all lines of “protocol” therapy are over and the patient is alive and progressing. The insidiousness of cancerous tumors lies in their variability. They mutate very quickly further, and adapt to any conditions, to any drugs. For the patient, this means the development of resistance - all drugs prescribed in the treatment protocols ceased to act on his tumor.
It is necessary to continue treatment - and the doctor has run out of “tools” prescribed by official treatment standards. There are other drugs, there is the right to prescribe them off-label, outside the standard lines of therapy. But how do you know which medicine to choose?
In this case, molecular genetic research gives us an understanding of which drug will be effective against this tumor, with this particular set of mutations. The appointment of such a drug allows you to win the main resource for the cancer patient - time.
Methodology Problems
Tumors are heterogeneous. They consist of different cells, which can differ quite significantly. And, for example, in 80% of tumor cells, a mutation of a certain gene is present, and 20% of the cells shared with a different distribution of chromosomes - and remained unmuted. Yes, we prescribe the drug according to the results of the molecular genetic test, and against 80% of the tumor cells it will work effectively, but for the remaining 20% it will be necessary to come up with another treatment.
Some types of cancer are more or less heterogeneous, for example, breast cancer. And some tumors, such as sarcomas, resemble vinaigrette in structure. This makes diagnosis and treatment difficult: it is impossible to know in advance in which part of the tumor which cells are, how many of their types, how much they differ. And you can’t, roughly speaking, take 10 samples from different places of the tumor - they will have to do 10 separate genetic studies.
Up to 30% of targeted and immunopreparations in Russia are prescribed without appropriate justification - without studies of tumor genetics. And part of these drugs turns out to be a waste of budget funds and patient money, because prescribing targeted treatment without understanding the genetics of the tumor is a tape measure: more than 600 drugs are registered. For example, for breast cancer, there are five treatment protocols, depending on the mutation of the HER2 / Neu gene.
In Western medicine, determining the genetic profile of a tumor is already becoming the standard of treatment. For Russian cancer patients, molecular genetic testing is still a rare case, unfortunately - for budget medicine it is still expensive. But there is hope that everything will change for the better. If now it costs 600 thousand rubles, then 5 years ago it cost more than a million - the technology is becoming easier and more advanced, and, therefore, more popular and affordable. Here time works for us.
Most oncologists in Russia do NOT use molecular genetic tests. Because they do not have sufficient experience with them and specific knowledge. It will not work just to open the report and "write off" the treatment from there. It is necessary to take into account many factors, to understand how all these numerous mutations affect each other, on tumor growth, on the patient’s potential individual tolerance to the drug, etc.
Therefore, it is not enough to make a genetic test, you need to be able to understand the results and draw the right conclusions. My colleagues and I most often first study the report ourselves (sometimes we have to sit at home in silence after work) - and then we also gather a consultation and make a collegial decision.
It is necessary to think through combinations of targeted drugs, be able to combine them with chemotherapeutic drugs, and consider possible side effects of such “cocktails”. This is a rather difficult task - and the doctor must be very motivated to constantly study.
But good patient stories, frankly, always motivate best.
Now we have a patient, 48 years old, with recurrent glioblastoma (an aggressive brain tumor). She came to us after undergoing two lines of therapy at the state cancer center. They did everything right there, performed radiation therapy and prescribed a targeted drug, but the tumor still returned. The woman was given six months of life.
We offered her full molecular genetic testing. Yes, it costs 600 thousand rubles, an abridged version, for 250, in her case did not fit - it was necessary to have extensive testing, with the most complete set of mutations.
But according to the results of the examination, she was prescribed a drug that is usually intended for the treatment of non-small cell lung cancer. It is effective against tumors with an EGRF mutation - in our patient, glioblastoma was with this mutation.
A woman comes to us to be treated and observed for 4 years. This is 5 times longer than with standard therapy. Moreover, she is independent, she has been living an ordinary life these 4 years, goes to work and is going to wait for her grandchildren.
So, even though we at Medicine 24/7 have to keep our brains in good shape all the time, to understand new and new studies of genetic mutations - the results are definitely worth it.
Be healthy.
All Articles