How mustard began to cure cancer (more or less)
Recently I accidentally talked in the clinic with a person suffering from leukemia, and this prompted me to write a little about one of the first, and so far the main, chemotherapeutic drugs for cancer treatment. And most importantly - how they were created.
But first, a little about mustards. Mustard is not one, there are several of them and I will tell about them all.
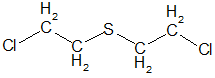
The first mustard gas, applied first by the Germans, and then by the Allies to the First World War, 2,2'-dichlorodiethylsulfide - had the formula - S (C2H4Cl) 2 or
The use of agents itself does not mean that they are simply “filled in” with the enemy by shelling, in fact, each agent has its own tactical niche, often very narrow. Tactical niche mustard - to make some terrain inaccessible or at least inaccessible for a relatively long period. Mustard gas is extremely poisonous, even a small drop of it remaining on the clothes will damage not only the owner of the clothes, but also anyone who will be with him in the same room. Moreover, its degassing is very laborious - mustard gas is dissolved and absorbed in almost all materials - from brick to rubber. In particular, this is the reason for the two-hour norm of staying in one gas mask on the ground contaminated with mustard gas. However, even in ideal conditions, it is not so easy to degas yprite - it rather slowly interacts with bleach solutions, its degassing requires mixing, since it is an oil-like substance. As an example, I cite the recollections of a certain N. M. Gogello from the book of L. Fedorov (the main person in Russia on the problems of chemical weapons):
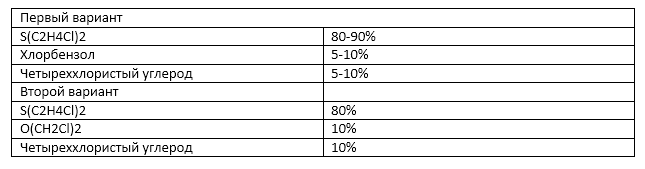
Of course, mustard gas was not used in its pure form, and it would be strange to expect an inscription on the shells of “Iperite”. According to the German nomenclature since the 1st world mustard gas was the main component of chemical shells “yellow cross” filled with a mixture of
At the same time, any sharply smelling wastes of organic synthesis enterprises were added to the mixtures - on the one hand, the “unfamiliar” agent significantly reduced the morale of the enemy soldiers, and on the other, it complicated the analysis of the mixture.
Because of the properties of mustard gas, the effectiveness of its use was very high - shells with it, of course, did not attack those enemy positions that their troops had to take, but those from which the enemy needed to be driven away. Or to infect a plot of terrain with yprite so that the enemy could not cross it. In a tactical sense, this is an exceptional opportunity. Let us recall the Soviet irrigation machines of the interwar epoch, which were designed on the basis of German equipment for infecting the localities of agents. The equipment, as well as a certain part of the technologies for the production of organic substances, were transferred to the USSR by the Weimar Republic in the late 1920s.

Mustard mustard is very, very poisonous. Although the data on its LD varies and may seem relatively low, for a HU the main result is the practical result of the application, which often drastically differs from the LD indicator. For example, botulism toxin with LD50 is according to current data 1.3-2.1 * 10-9 g / kg considered to be the strongest poison. At VX LD50 only 70 * 10-6 g / kg i.e. the difference would seem to be more than four orders of magnitude. At the same experiments, it turned out that when infecting a locality, botulinum toxin is inferior in efficiency to VX. Therefore, we can conclude that mustard gas used the title of "king of poisons" in the 1920s not without reason. For mustard gas, the main property is a bright cytotoxic effect - the ability to cause the death of body cells with which it comes into contact. Contact with mustard on the skin causes severe necrosis, in the area of the joints - lesions, resulting in loss of mobility of the joint, in the eyes - blindness, oral administration of mustard gas - generally speaking, death, etc. A modern view of the mechanism of action of mustard is that it alkylates DNA, damaging it, as a result of which a self-destruct mechanism is activated in the cell. Very schematically, the reaction looks like this:

As you can see, the mustard molecule is isomerized into a cation which is a Lewis acid, therefore, there is nothing unusual in its reaction with the Lewis base - the amino group in the DNA - no.
Wikipedia says that mustard is an alkylating agent, which from a chemical point of view is not quite true. The fact is that with the usual chemical reactions of its alkylating action, it almost does not show - it reacts with ammonia only when heated in a sealed ampoule, with amines - in the presence of a weak base, it interacts very badly with amino acids (which are the basis of proteins). Reactions are more or less active only with secondary amines. So it can be said that there was lucky with mustard gas - it is sufficiently active for DNA damage, but not too active to lose the opportunity to get into the cell itself, having undergone hydrolysis, plus its convenient physical properties (high boiling liquid). Because of this, the efforts of chemists in many countries in the interwar period were devoted to the synthesis of mustard gas, the search for its more toxic counterparts and the improvement of operational properties. It was precisely in the sense of poisonousness that an unpleasant surprise awaited - many substances with cytotoxic properties similar to mustard gas were found, but the mustard gas turned out to be an almost exclusive poison. In reality, only so-called oxygen mustard gas and nitrogen mustard gas.
Oxygen mustard with formula
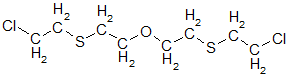
was obtained immediately in a mixture with ordinary mustard by the action of concentrated hydrochloric acid on thiodiglycol at elevated temperatures (UK). Nothing noticeable in history, he has not distinguished himself.
What can not be said about nitrogenous mustard gas, the development of which was most actively conducted in the USA (although in Germany at the end of the war about 2 thousand tons of nitrogen mustard were found, which is not very much by the standards of organic matter). Recall the formula of mustard gas at the beginning. And the tris (2-chloroethyl) amine completely similar to it, although it was studied in detail only in the mid-1930s
The effect of this substance was very similar to the effect of mustard gas, moreover, it even had certain advantages over it. In particular, it was much more resistant to oxidation, therefore, it was more difficult to degass it than mustard gas. Industrial synthesis was carried out by chlorination of triethanolamine
N (C2H4OH) 3 + 3SOCl2 = N (C2H4Cl) 3 + 3SO2 + 3HCl
If someone noticed, then triethanolamine is part of many fluxes for soldering, for example LTI-120. As far as I know, although triethanolamine is included in the list of precursors for the production of chemical agents, anyone can buy it. Like thiodiglycol, by the way, the main raw material for the production of regular mustard. Speaking of triethanolamine, it can be noted that it is certainly very, very far from nitrogenous mustard gas in terms of its toxicity, however, it is also impossible to call it absolutely harmless and it requires careful handling.
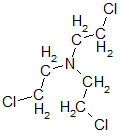
Returning to nitrogen mustard - research then showed that the most significant is the presence of two groups —CH2CH2Cl on one nitrogen atom; the third group may be different. Therefore, two more nitrogen mustard bis (2-chloroethyl) ethylamine
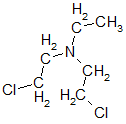
and bis (2-chloroethyl) methylamine
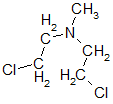
In parallel, studies on chloroethanolamines as an OV, Yale University (USA) conducted research on their same medical use. In those days, radiotherapy was also just being born, it is clear that it is not difficult to draw a parallel between the effect of ionizing radiation on the body and the effect of mustard in both cases, the symptoms are somewhat similar. This probably prompted A. Gilman and L. Goodman to try to treat cancer with mustard. The first drugs they used in the early 1940s were exactly three nitrogen mustards, probably the first drug was bis (2-chloroethyl) methylamine called chlormethine, mechlorethamine, and later in the USSR embikhin. Although this substance still finds application in medicine, one should not expect that then a miracle happened - initially the tumors disappeared, but then returned again, and with a loss of sensitivity to chlormethine.
However, this drug still later found its niche in the field of leukemia treatment. Even a not completely positive result was a breakthrough at that time, a somewhat late publication caused a wave of research in the field of chemotherapeutic anticancer drugs, especially derivatives of nitrogen mustard. As a result, an enormous amount of drugs were synthesized with the same group –N (C2H4Cl) 2 or = N (C2H4Cl), which had a more significant effect on the tumor and a slightly less destructive effect on the body. However, the hazardous properties of nitrogen mustard were preserved.
Interestingly, all mustards are carcinogenic - due to DNA damage of healthy cells, they are really able to provoke cancer. Yperity is included in the 1st group of carcinogens from IARC, i.e. a group of substances whose carcinogenic effect on humans has been proven. Tamoxifen, a widely used anti-cancer agent, is also on the same list. How does it happen that the drugs that cure cancer by themselves cause it? This is only an apparent paradox, everything is absolutely logical - if the substance causes the death of ordinary cells, then it will certainly cause the death of cancer cells, the more they are more “tender”.
Once I accidentally came across an English-language video in the format of questions and answers about cancer (or just tumors), which I probably would not remember if there wasn’t a question like “Why not take a cure for cancer by analogy with vitamins to avoid it?” ? "LOL. Now it is clear to the reader that the use of such drugs on a healthy person will at least worsen his health, may cause cancer, and an overdose will simply kill, as mustard gas kills.
Well, for the sake of interest, I cite formulas of chemotherapeutic anticancer drugs in which the reader can easily find the basis of nitrogen mustard:

Uramustin, chloroethylaminouracil, uracil mustard, dopan
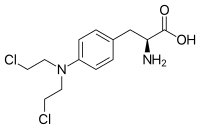
Melphalan "Alkeran", "Sarkolizin"

Chlorambucil

Bendamustine
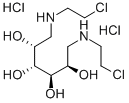
Mannomustine, Degranol

Prednimustin

Estramustin

Novambihin

Pafenzil

Lofenal

Cyclophosphamide

Ifosfamide

Mafosfamide

Trofosfamide, ixoten

Perphosphamide, Pergamid

Hydroxycyclophosphamide

Aldophosphamide

Gluphosphamide
Etc.
Summarizing the above, you can ask how many people died as a result of the use of mustard gas (on the spot, from the consequences, during the production of mustard gas, in the process of its utilization, etc.) and how many additional years were received, sometimes decades, as a result of chemotherapy mustard preparations? Personally, I am sure that the number of the second has long since exceeded the number of the first, so that in this case the adage “a blessing in disguise” works. For example, if the use of skin-resistant agents during World War I had not been used, the use of anticancer drugs would have moved back several decades ahead, maybe even for a longer period. The same happened with the chemistry of arsenic - the search for new agents based on arsenic gave a powerful impetus to chemotherapeutic drugs for syphilis (however, the chemistry of arsenic-organic compounds developed in the pre-war period).
But first, a little about mustards. Mustard is not one, there are several of them and I will tell about them all.
The first mustard gas, applied first by the Germans, and then by the Allies to the First World War, 2,2'-dichlorodiethylsulfide - had the formula - S (C2H4Cl) 2 or

The use of agents itself does not mean that they are simply “filled in” with the enemy by shelling, in fact, each agent has its own tactical niche, often very narrow. Tactical niche mustard - to make some terrain inaccessible or at least inaccessible for a relatively long period. Mustard gas is extremely poisonous, even a small drop of it remaining on the clothes will damage not only the owner of the clothes, but also anyone who will be with him in the same room. Moreover, its degassing is very laborious - mustard gas is dissolved and absorbed in almost all materials - from brick to rubber. In particular, this is the reason for the two-hour norm of staying in one gas mask on the ground contaminated with mustard gas. However, even in ideal conditions, it is not so easy to degas yprite - it rather slowly interacts with bleach solutions, its degassing requires mixing, since it is an oil-like substance. As an example, I cite the recollections of a certain N. M. Gogello from the book of L. Fedorov (the main person in Russia on the problems of chemical weapons):
“My father, an artilleryman by training, served after the revolution as head of the artillery warehouse in Odessa, and in 1923 he was seconded to Moscow, where he was assigned to serve in the chemical landfill in Kuzminki. Soon, dad got a mustard defeat: when he opened the projectile, a drop fell on his boot, he did not notice, and he didn’t know, probably, what it was. For three months he sat on the veranda (it was summer) with an extended leg, on the rise of which there was a terrible ulcer. I heard snatches of conversations with colleagues, who visited him and discussed the insidious properties of mustard gas. Later, my father told me that the task of him and his colleagues was to deal with the captured shells - their design and what they were stuffed with. In parallel with the service at the chemical pioneer, my father studied at the chemical faculty of Moscow State Technical University (in those years there was a chemical faculty). ”
Of course, mustard gas was not used in its pure form, and it would be strange to expect an inscription on the shells of “Iperite”. According to the German nomenclature since the 1st world mustard gas was the main component of chemical shells “yellow cross” filled with a mixture of

At the same time, any sharply smelling wastes of organic synthesis enterprises were added to the mixtures - on the one hand, the “unfamiliar” agent significantly reduced the morale of the enemy soldiers, and on the other, it complicated the analysis of the mixture.
Because of the properties of mustard gas, the effectiveness of its use was very high - shells with it, of course, did not attack those enemy positions that their troops had to take, but those from which the enemy needed to be driven away. Or to infect a plot of terrain with yprite so that the enemy could not cross it. In a tactical sense, this is an exceptional opportunity. Let us recall the Soviet irrigation machines of the interwar epoch, which were designed on the basis of German equipment for infecting the localities of agents. The equipment, as well as a certain part of the technologies for the production of organic substances, were transferred to the USSR by the Weimar Republic in the late 1920s.

Mustard mustard is very, very poisonous. Although the data on its LD varies and may seem relatively low, for a HU the main result is the practical result of the application, which often drastically differs from the LD indicator. For example, botulism toxin with LD50 is according to current data 1.3-2.1 * 10-9 g / kg considered to be the strongest poison. At VX LD50 only 70 * 10-6 g / kg i.e. the difference would seem to be more than four orders of magnitude. At the same experiments, it turned out that when infecting a locality, botulinum toxin is inferior in efficiency to VX. Therefore, we can conclude that mustard gas used the title of "king of poisons" in the 1920s not without reason. For mustard gas, the main property is a bright cytotoxic effect - the ability to cause the death of body cells with which it comes into contact. Contact with mustard on the skin causes severe necrosis, in the area of the joints - lesions, resulting in loss of mobility of the joint, in the eyes - blindness, oral administration of mustard gas - generally speaking, death, etc. A modern view of the mechanism of action of mustard is that it alkylates DNA, damaging it, as a result of which a self-destruct mechanism is activated in the cell. Very schematically, the reaction looks like this:

As you can see, the mustard molecule is isomerized into a cation which is a Lewis acid, therefore, there is nothing unusual in its reaction with the Lewis base - the amino group in the DNA - no.
Wikipedia says that mustard is an alkylating agent, which from a chemical point of view is not quite true. The fact is that with the usual chemical reactions of its alkylating action, it almost does not show - it reacts with ammonia only when heated in a sealed ampoule, with amines - in the presence of a weak base, it interacts very badly with amino acids (which are the basis of proteins). Reactions are more or less active only with secondary amines. So it can be said that there was lucky with mustard gas - it is sufficiently active for DNA damage, but not too active to lose the opportunity to get into the cell itself, having undergone hydrolysis, plus its convenient physical properties (high boiling liquid). Because of this, the efforts of chemists in many countries in the interwar period were devoted to the synthesis of mustard gas, the search for its more toxic counterparts and the improvement of operational properties. It was precisely in the sense of poisonousness that an unpleasant surprise awaited - many substances with cytotoxic properties similar to mustard gas were found, but the mustard gas turned out to be an almost exclusive poison. In reality, only so-called oxygen mustard gas and nitrogen mustard gas.
Oxygen mustard with formula

was obtained immediately in a mixture with ordinary mustard by the action of concentrated hydrochloric acid on thiodiglycol at elevated temperatures (UK). Nothing noticeable in history, he has not distinguished himself.
What can not be said about nitrogenous mustard gas, the development of which was most actively conducted in the USA (although in Germany at the end of the war about 2 thousand tons of nitrogen mustard were found, which is not very much by the standards of organic matter). Recall the formula of mustard gas at the beginning. And the tris (2-chloroethyl) amine completely similar to it, although it was studied in detail only in the mid-1930s

The effect of this substance was very similar to the effect of mustard gas, moreover, it even had certain advantages over it. In particular, it was much more resistant to oxidation, therefore, it was more difficult to degass it than mustard gas. Industrial synthesis was carried out by chlorination of triethanolamine
N (C2H4OH) 3 + 3SOCl2 = N (C2H4Cl) 3 + 3SO2 + 3HCl
If someone noticed, then triethanolamine is part of many fluxes for soldering, for example LTI-120. As far as I know, although triethanolamine is included in the list of precursors for the production of chemical agents, anyone can buy it. Like thiodiglycol, by the way, the main raw material for the production of regular mustard. Speaking of triethanolamine, it can be noted that it is certainly very, very far from nitrogenous mustard gas in terms of its toxicity, however, it is also impossible to call it absolutely harmless and it requires careful handling.
Returning to nitrogen mustard - research then showed that the most significant is the presence of two groups —CH2CH2Cl on one nitrogen atom; the third group may be different. Therefore, two more nitrogen mustard bis (2-chloroethyl) ethylamine

and bis (2-chloroethyl) methylamine
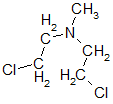
In parallel, studies on chloroethanolamines as an OV, Yale University (USA) conducted research on their same medical use. In those days, radiotherapy was also just being born, it is clear that it is not difficult to draw a parallel between the effect of ionizing radiation on the body and the effect of mustard in both cases, the symptoms are somewhat similar. This probably prompted A. Gilman and L. Goodman to try to treat cancer with mustard. The first drugs they used in the early 1940s were exactly three nitrogen mustards, probably the first drug was bis (2-chloroethyl) methylamine called chlormethine, mechlorethamine, and later in the USSR embikhin. Although this substance still finds application in medicine, one should not expect that then a miracle happened - initially the tumors disappeared, but then returned again, and with a loss of sensitivity to chlormethine.
However, this drug still later found its niche in the field of leukemia treatment. Even a not completely positive result was a breakthrough at that time, a somewhat late publication caused a wave of research in the field of chemotherapeutic anticancer drugs, especially derivatives of nitrogen mustard. As a result, an enormous amount of drugs were synthesized with the same group –N (C2H4Cl) 2 or = N (C2H4Cl), which had a more significant effect on the tumor and a slightly less destructive effect on the body. However, the hazardous properties of nitrogen mustard were preserved.
Interestingly, all mustards are carcinogenic - due to DNA damage of healthy cells, they are really able to provoke cancer. Yperity is included in the 1st group of carcinogens from IARC, i.e. a group of substances whose carcinogenic effect on humans has been proven. Tamoxifen, a widely used anti-cancer agent, is also on the same list. How does it happen that the drugs that cure cancer by themselves cause it? This is only an apparent paradox, everything is absolutely logical - if the substance causes the death of ordinary cells, then it will certainly cause the death of cancer cells, the more they are more “tender”.
Once I accidentally came across an English-language video in the format of questions and answers about cancer (or just tumors), which I probably would not remember if there wasn’t a question like “Why not take a cure for cancer by analogy with vitamins to avoid it?” ? "LOL. Now it is clear to the reader that the use of such drugs on a healthy person will at least worsen his health, may cause cancer, and an overdose will simply kill, as mustard gas kills.
Well, for the sake of interest, I cite formulas of chemotherapeutic anticancer drugs in which the reader can easily find the basis of nitrogen mustard:

Uramustin, chloroethylaminouracil, uracil mustard, dopan

Melphalan "Alkeran", "Sarkolizin"

Chlorambucil

Bendamustine

Mannomustine, Degranol

Prednimustin

Estramustin

Novambihin

Pafenzil

Lofenal

Cyclophosphamide

Ifosfamide

Mafosfamide

Trofosfamide, ixoten

Perphosphamide, Pergamid

Hydroxycyclophosphamide

Aldophosphamide

Gluphosphamide
Etc.
Summarizing the above, you can ask how many people died as a result of the use of mustard gas (on the spot, from the consequences, during the production of mustard gas, in the process of its utilization, etc.) and how many additional years were received, sometimes decades, as a result of chemotherapy mustard preparations? Personally, I am sure that the number of the second has long since exceeded the number of the first, so that in this case the adage “a blessing in disguise” works. For example, if the use of skin-resistant agents during World War I had not been used, the use of anticancer drugs would have moved back several decades ahead, maybe even for a longer period. The same happened with the chemistry of arsenic - the search for new agents based on arsenic gave a powerful impetus to chemotherapeutic drugs for syphilis (however, the chemistry of arsenic-organic compounds developed in the pre-war period).
All Articles