Neurogenesis: physiological basis and perspectives of regulation for therapeutic purposes
Neurogenesis is a multistep process of formation of new nerve cells in the mature central nervous system (CNS), which is its adaptive function.
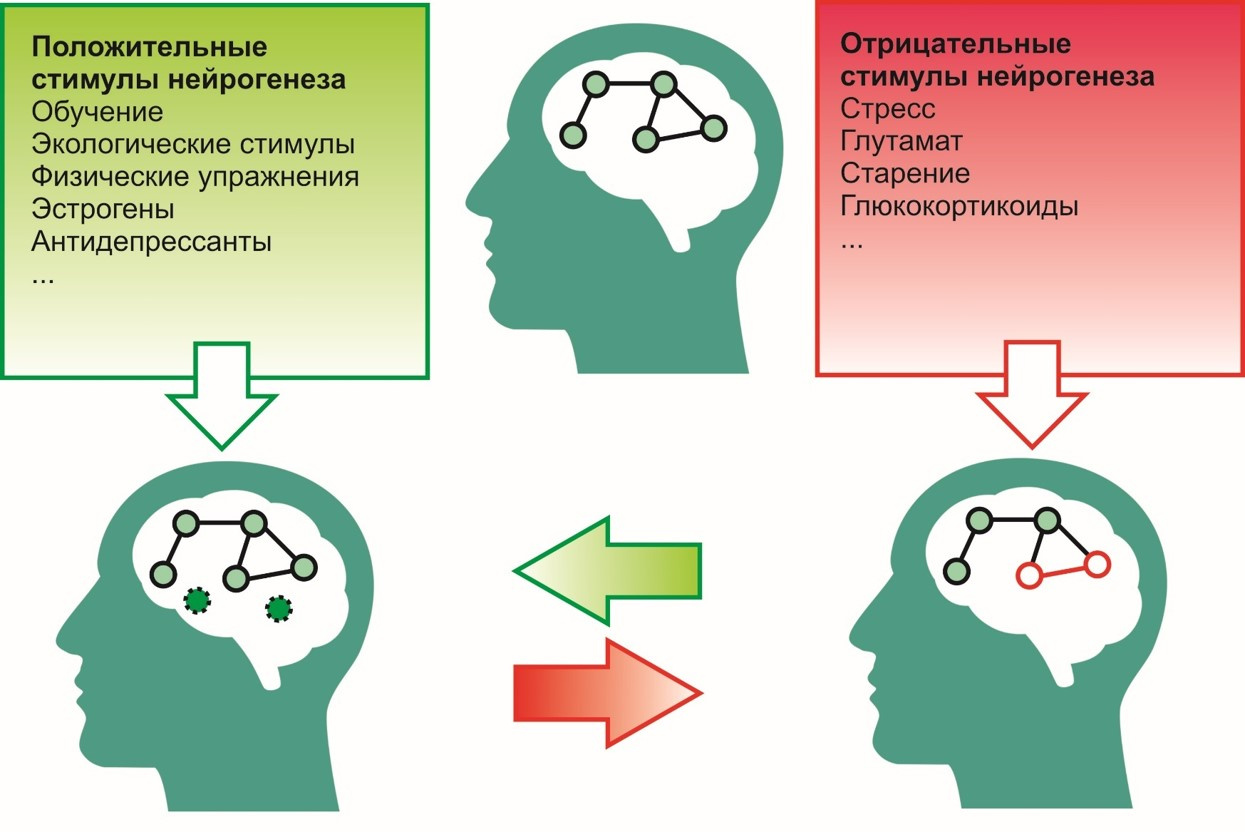
To positive incentives, i.e. stimuli that contribute to the process of neurogenesis include:
To negative -
For many years, central dogmas existed in neuroscience, which did not allow the very possibility of neurogenesis.

The ideas about the absence of neurogenesis in the brain of mature vertebrates were based on four principles:
Stages of the formation of ideas about the neurogenesis of the mature brain
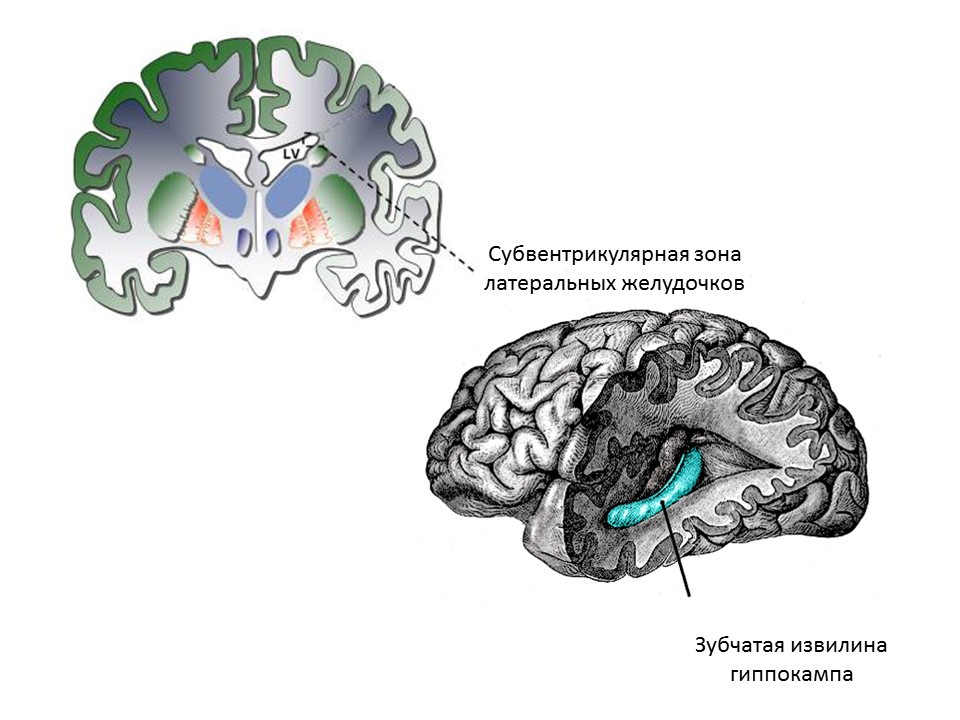
The main neurogenic zones of the adult (or mature) brain are the dentate gyrus of the hippocampus and the subventricular zone of the lateral ventricles. Zones are shown.
Stages of neurogenesis in the dentate gyrus of the hippocampus of the mature brain
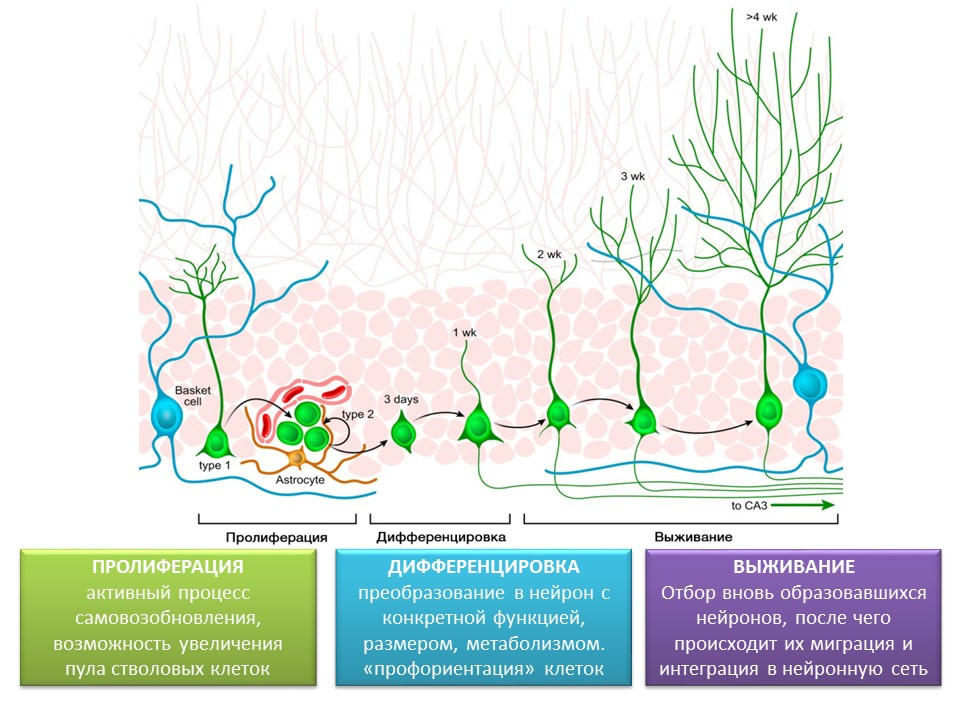
Proliferation - the active process of self-renewal, the possibility of increasing the pool of stem cells.
Differentiation - transformation into a neuron with a specific function, size, metabolism. In fact, the "career guidance" of future nerve cells.
Survival is the selection of newly formed neurons, after which they migrate and integrate into the neural network. The selection of nerve cells is apoptosis (programmed death) of the part of the formed neurons. The remaining cells migrate to the appropriate area of the brain and integrate into the neural network.
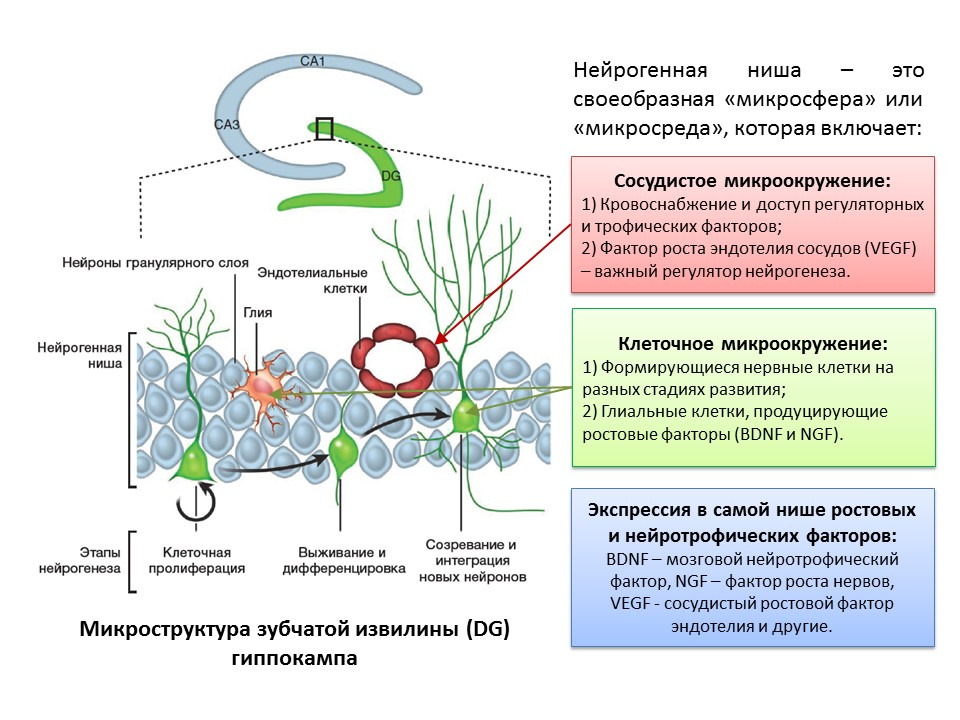
The term “neurogenic niche” is inseparably connected with the term “neurogenesis”. By itself, "neurogenic niche" is a "microsphere" in which the process of neurogenesis itself takes place. Neurogenic niche includes:
An important and interesting issue that deserves special attention is the question of how to detect neurogenesis in the brain tissues of mature mammals.
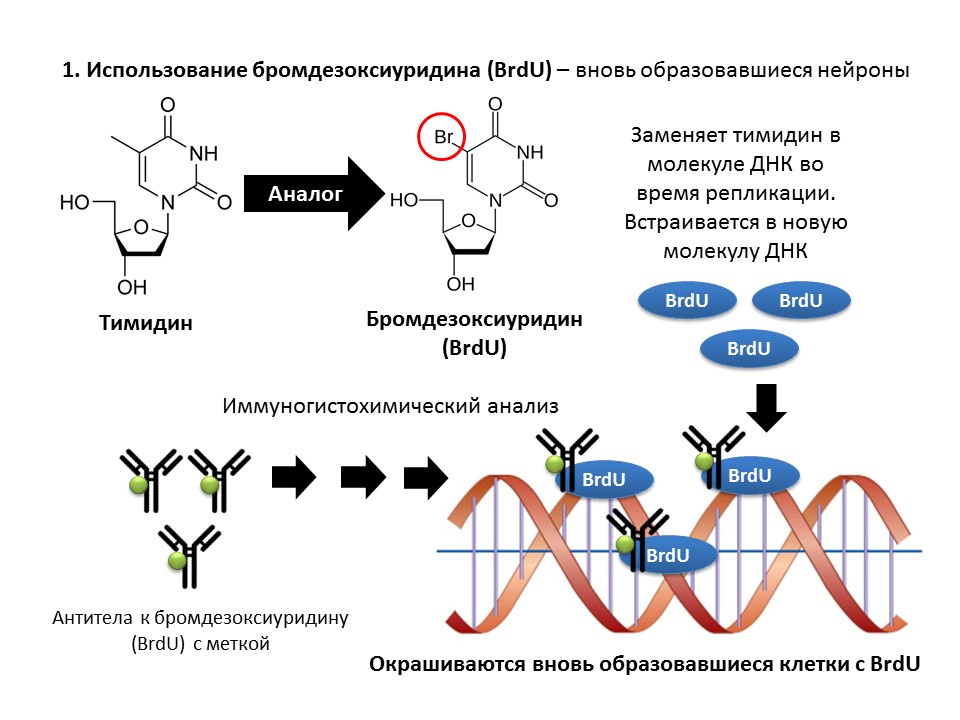
Bromodeoxyuridine (BrdU) is a thymidine structural analogue - a component of the DNA molecule. When introduced into the body, BrdU is inserted into the DNA of dividing cells instead of thymidine, providing an opportunity to detect the newly formed cells and separate them from the “old” ones. After receiving a sample of brain tissue, they are treated with antibodies to BrdU (antibodies contain a fluorescent label), which bind to BrdU via the mechanism of the immunochemical “antigen-antibody” reaction and provide an opportunity for the colorimetric determination of BrdU. Thus, it is possible to quantify BrdU-labeled cells, so-called BrdU positive cells, on the microslide.
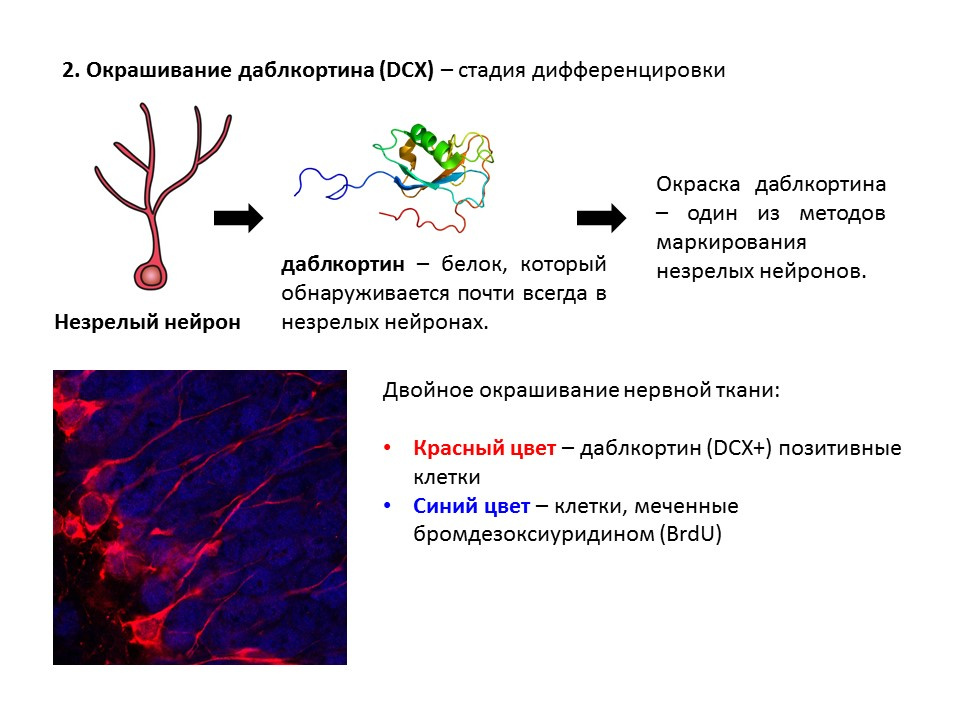
Doublecortin is a protein that is almost always found in immature neurons and allows them to be detected.
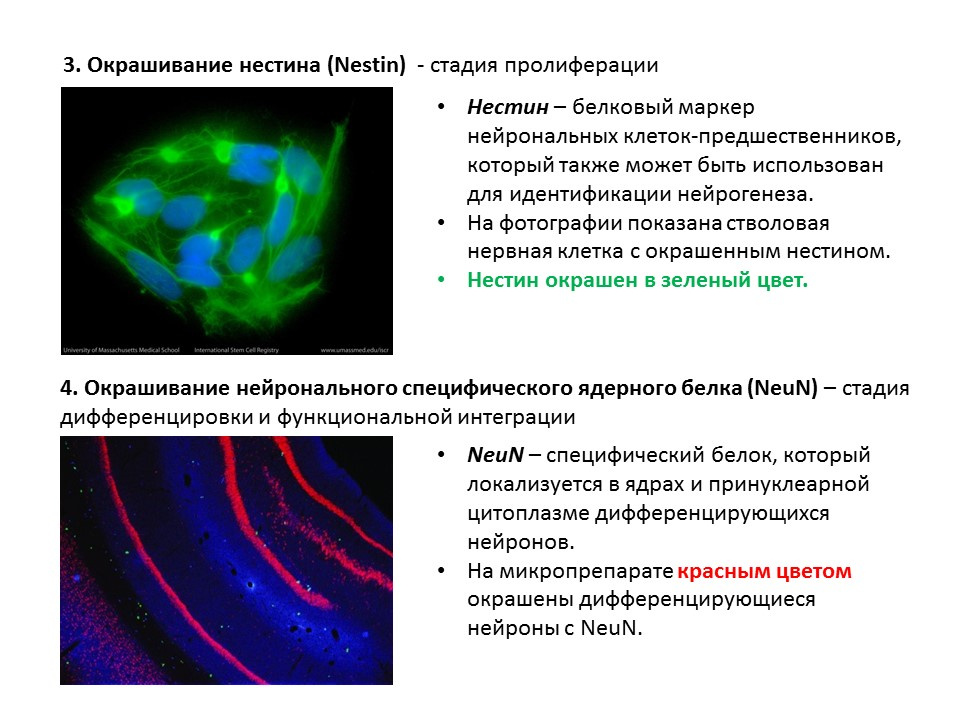
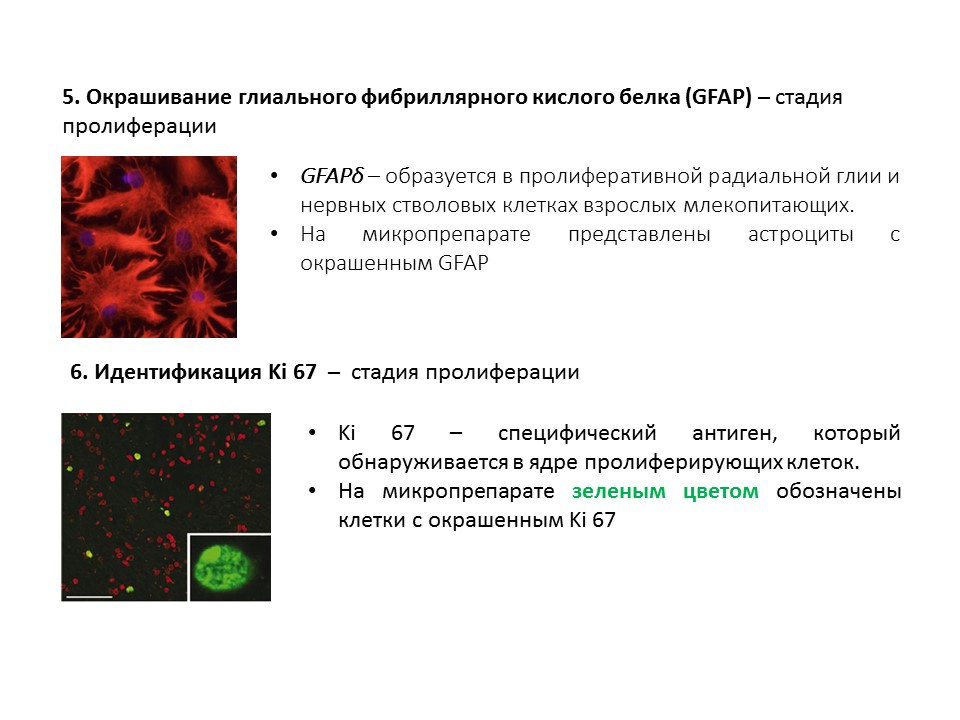
A summary diagram of the main methods for detecting the various stages of neurogenesis, which summarizes the presented early information.
The concept of an “enriched environment” is associated with environmental factors regulating the processes of neurogenesis. The term “enriched environment” itself includes a favorable habitat, namely the presence of a sufficient amount of food, a comfortably arranged space and the possibility for free search activity.
DOI: 10.1002 / hipo.22218
DOI: 10.1038 / 386493a0
DOI: 10.1016 / j.brainres.2011.08.007
DOI: 10.1016 / j.neuroscience.2011.10.040
PMID: 9547229
In animal experiments, it was found that being in an “enriched environment” has a positive effect on neurogenesis: the production of growth factors and neurotrophins, the number of proliferating cells and their survival increase. Increased neurogenesis is correlated with improved cognitive function in animals (mainly with learning and memory processes).
The maintenance of animals in conditions of social isolation in the laboratory, by contrast, acted as a negative regulator of neurogenesis.
Moreover, placing animals in an “enriched environment” after cerebral ischemia contributed to enhancing the regenerative processes in the neurogenic zones of the brain.
The results of such studies are not something extraordinary, since modern medicine uses rehabilitation courses and recreational rest.
An important role in the regulation of neurogenesis is played by the neurotransmitters of the CNS. The picture shows a summary of such a regulation.
DOI: 10.1152 / physrev.00004.2014
The regulatory role of the presented CNS neurotransmitters in the processes of neurogenesis correlates with a deficiency or excess of these molecules in various diseases of the CNS associated with changes in the level of neurogenesis.
Due to the possibility of pharmacological manipulation of the neurotransmitter system of the CNS, we can evaluate the contribution of a neurotransmitter to different stages of neurogenesis of the mature brain.
Fluoxetine (an antidepressant from the group of selective serotonin reuptake inhibitors) that can increase the concentration of serotonin in the CNS has been found to increase the proliferation of nerve stem cells in the dentate gyrus of the hippocampus of rodents and primates. Later, on the stem cells themselves, serotonin receptors 1A of the subtype (5-HT1A receptors) were detected and this is consistent with the ability of 8-OH-DPAT (selective activator of these receptors) to stimulate the proliferation and survival of new neurons in the dentate gyrus of experimental animals (mice and rats).
The presented experiments confirm the important regulatory role of serotonin in the processes of neurogenesis in the adult brain.
DOI: 10.4161 / cc.8.18.9512
DOI: 10.1016 / j.neuropharm.2011.01.026
Dopamine is also actively involved in the regulation of neurogenesis. It was shown that activation of dopamine receptors 1 and 2 of type (D1 and D2) by bromocriptine leads to an increase in the differentiation process of neural stem cells, while the stimulation of D3 receptors by pramipexol leads to an increase in proliferation.
Norepinephrine is able to increase the number of precursors of neural stem cells through the activation of beta-3 adrenoreceptors. This effect was shown using a selective agonist (activator) of beta-3-adrenergic receptors - the substance BRL37344.
The regulatory role of norepinephrine in processes of mature neurogenesis was evaluated in a comprehensive in vitro study (cell culture) in vivo (in mice). Adding norepinephrine to cell culture resulted in an increase in the size of neurospheres (clusters of neural stem cells). Systemic administration of a selective beta-3 adrenoreceptor agonist resulted in increased proliferation of neural stem cells in the hippocampus of mice. These studies confirm data on the presence of adrenoreceptors on immature nerve cells, and also indicate the regulatory role of norepinephrine, which, apparently, is realized through beta-3-adrenoreceptors.
DOI: 10.1523 / JNEUROSCI.3780-09.2010
Nerve stem cells, as mentioned earlier, are capable of producing regulation factors themselves. One of these factors is gamma-aminobutyric acid (GABA) - the main inhibitory mediator of the central nervous system. In particular, in the culture of mouse neural cell progenitors, it was shown that neural stem cells enhance GABA synthesis, which in turn enhances proliferation, activating radial glia (gives rise to stem cells) and promotes an increase in the size of neurospheres (accumulation of stem cells) in the experiment.
It was found that this effect of GABA is realized through GABA-B receptors. In studies, a selective GABA-B receptor agonist, baclofen, was used.
DOI: 10.1002 / jcp.21422
Nakamichi N, Takarada T, Yoneda Y. Neurogenesis mediated by gamma-aminobutyric acid and glutamatesignaling. J. Pharmacol Sci. 2009; 110 (2): 133-49
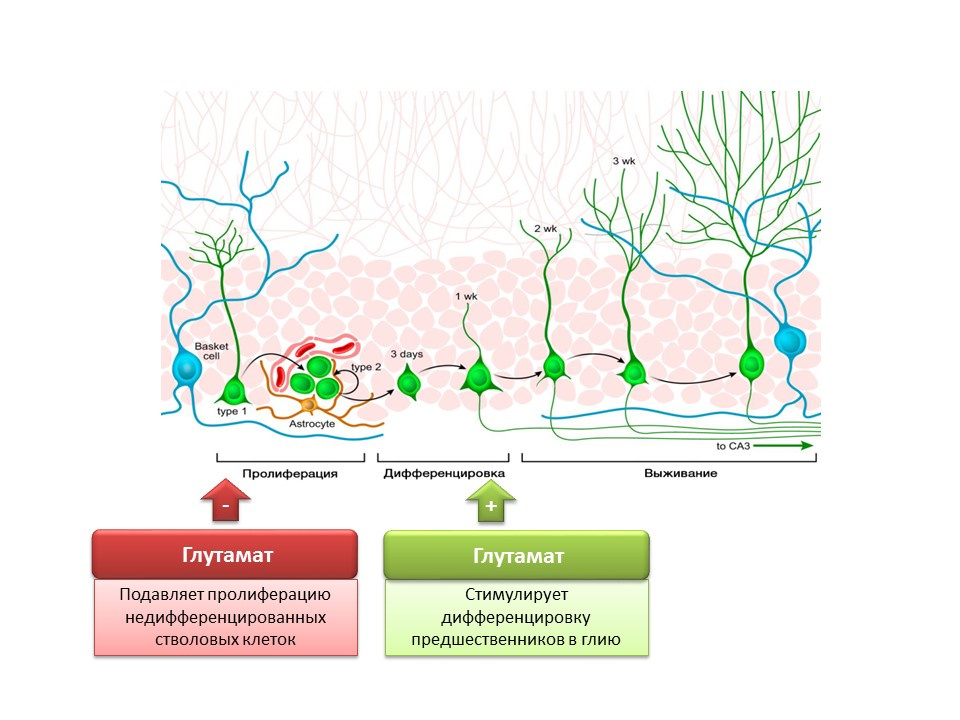
The role of the main excitatory mediator in the central nervous system is twofold. On the one hand, glutamate inhibits the proliferation of undifferentiated stem cells. On the other hand, it stimulates the differentiation of progenitors into the glial line. It is believed that certain ratios of glutamate and GABA are necessary for regulating the differentiation of neural stem cells into certain types of neurons. For example, glutamate, as already mentioned, promotes the formation of glial cells (astrocytes), and GABA contributes to the formation of neurons that synthesize GABA itself (GABAergic neurons).
Kotani S et al. H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis.Chem Biol Interact. 2008; 175 (1-3): 227-30
Kita Y et al. Galantamine promotes adult hippocampal neurogenesis via muscine and α7 nicotinic receptors in mice. Int J Neuropsychopharmacol.2014; 17 (12): 1957-68.
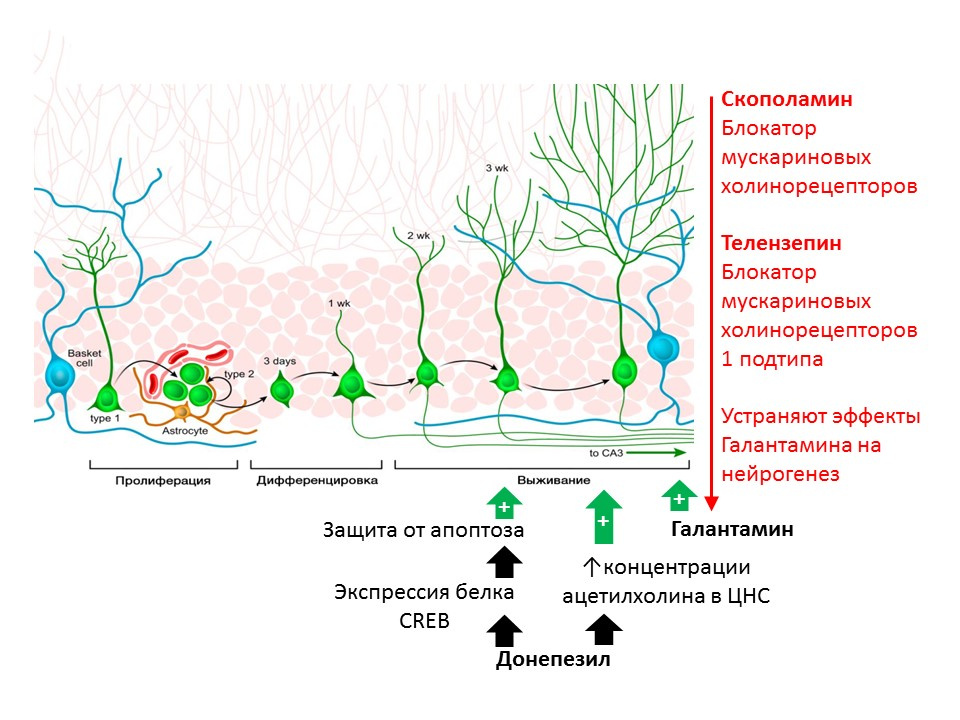
The regulatory role of acetylcholine (ACh) is shown in rats with substances that prevent its destruction (acetylcholinesterase inhibitors - an enzyme that cleaves Ac5). Donepezil and galantamine increase the concentration of ACh in the CNS (donepezil also increases the expression of the CREB protein - a transcription factor, thereby enhancing the protection of immature neurons from apoptosis) and increasing the survival of immature neurons. The positive effect of galantamine on neurogenesis is eliminated by scopolamine (a blocker of all muscarinic cholinoreceptor subtypes) and telenzepine (blocker 1 of muscarinic cholinoreceptor subtypes), which suggests the implementation of ACh on neurogenesis through the activation of muscarinic receptors of type 1 ( Kotani S et al. H. inhibitor, enhances adult hippocampal neurogenesis, Chem Biol Interact. 2008; 175 (1-3): 227-30; Int. Neuropsychopharmacol 2014; 17 (12): 1957-68. ).
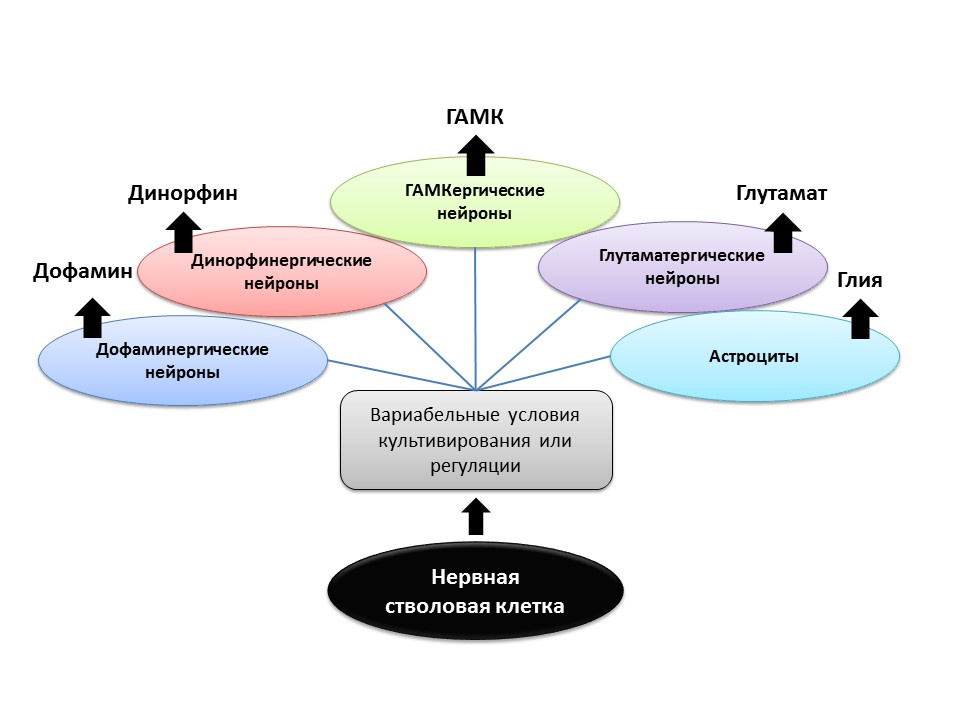
Under experimental conditions (in animals or in cell culture), it was shown that changing the ratio of regulatory factors and the addition of neurotransmitters themselves to neural stem cells allows one to “program” the neurotransmitter phenotype (that is, the function of the future cell) of newly formed neurons. For example, the cultivation of stem cells with astrocytes allows you to get a population of glutamatergic neurons (neurons that produce glutamate), and with dopamine - dopaminergic.
It is possible that such a directed effect in the future can be used for the selective formation of nerve cells of a particular phenotype. For example, if we know which type is lost in a particular disease (in Parkinson's disease, dopaminergic die, in Alzheimer's disease, cholinergic). But at present, this aspect of neurogenesis remains unexplored until the end, and it is still too early to talk about programmed regulation of neurogenesis (there are isolated studies).
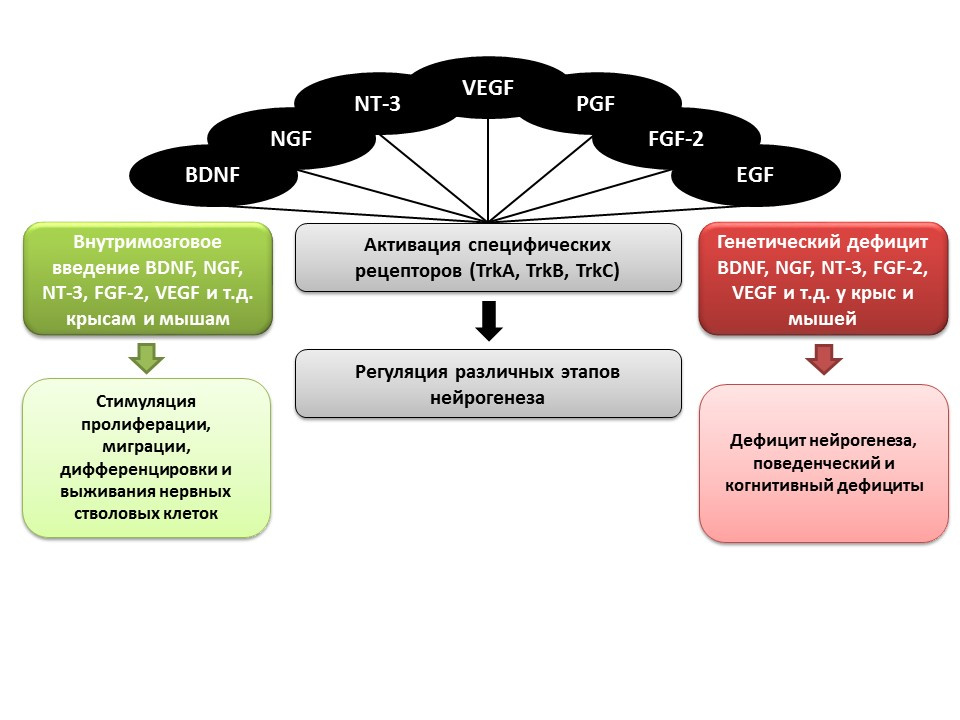
An important role in the regulation of neurogenesis, as mentioned earlier, is played by growth and neurotrophic factors, including:
In animal experiments (rats and mice), it was shown that intracerebral administration of these factors contributes to the enhancement of all stages of neurogenesis. Congenital deficiency (transgenic mice with knockout genes of relevant factors) leads to impaired neurogenesis, as well as behavioral and cognitive deficits. These data confirm the important regulatory role of these factors in neurogenesis.
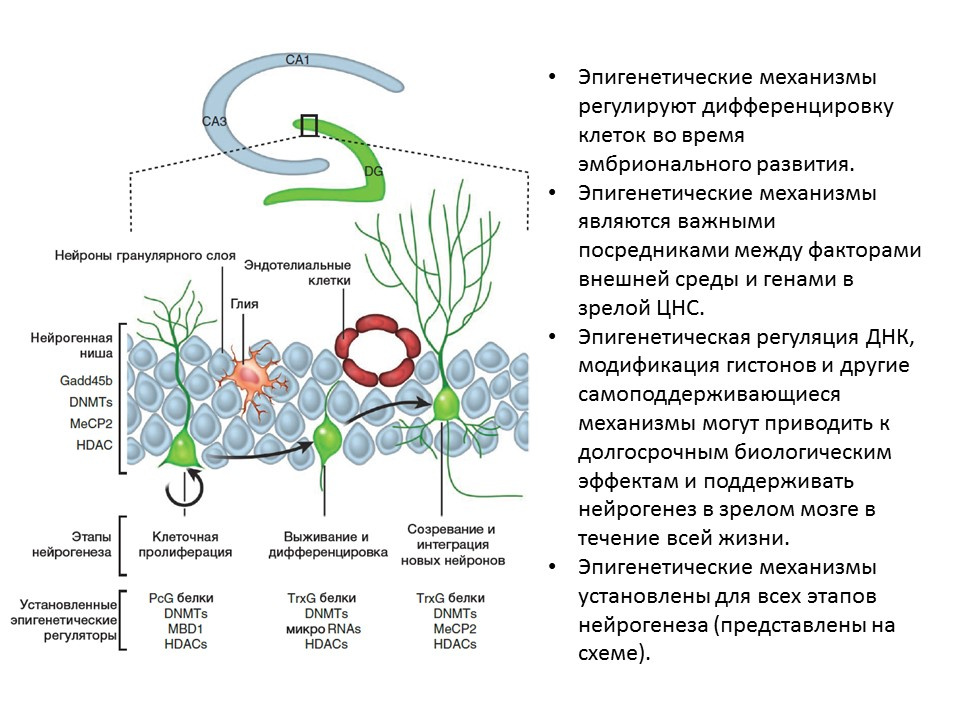
Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci.2010; 13 (11): 1338-44.
For all stages of neurogenesis, epigenetic factors of regulation have been established, for example:
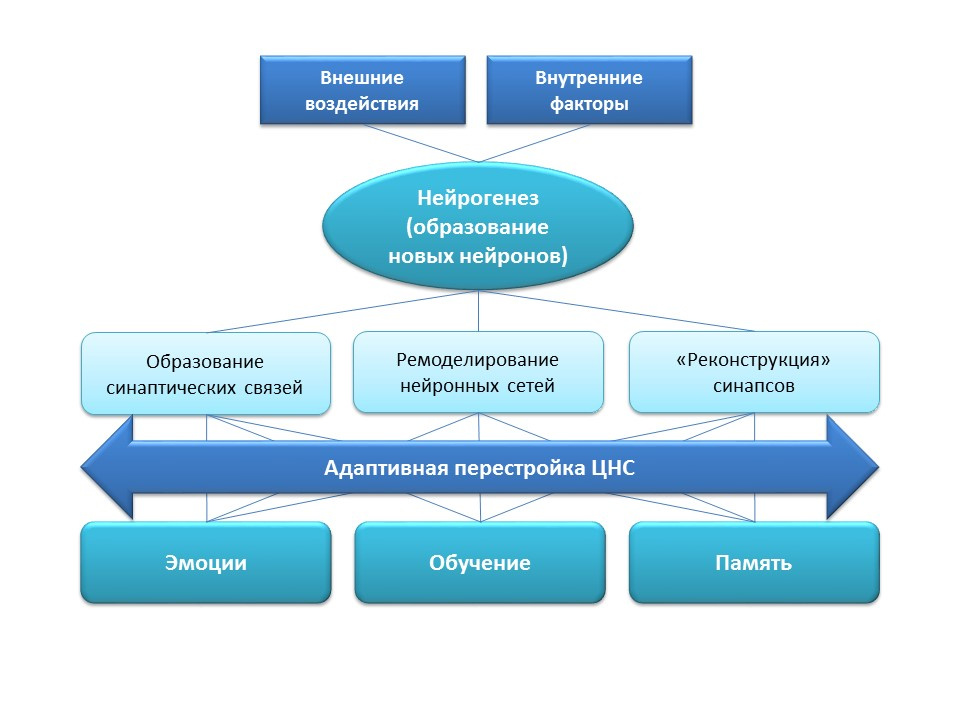
Neurogenesis has an important adaptation function in the central nervous system, which is the formation of new synaptic connections (with the participation of new nerve cells), remodeling (restructuring) of existing neural networks depending on the effects of external factors (training, physical activity, stress, etc.), "Reconstruction" of lost synaptic connections (under the influence of external and internal factors).
All the listed adaptive changes have a direct impact on the emotional response, learning processes and memory. Neurogenesis helps the nervous system to maintain "plasticity", to change and rebuild under the new conditions and objectives.
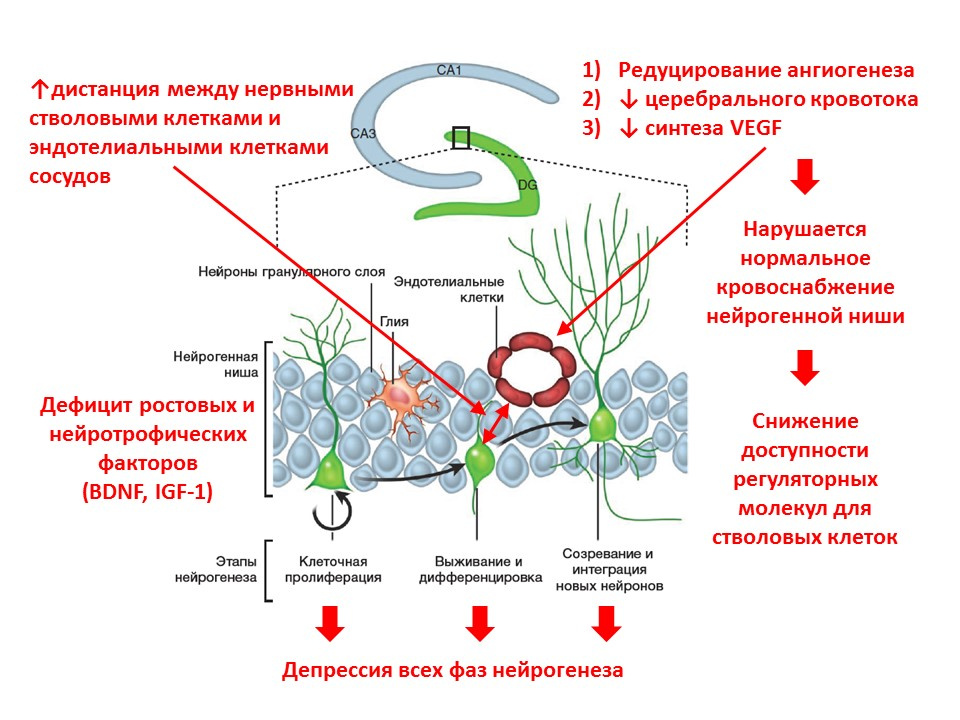
With age, the processes of neurogenesis begin to fade. At the molecular level, aging is accompanied by the following changes in neurogenesis:
However, age-related changes in neurogenesis are not irreversible, and the adaptive function of the brain can be stimulated.
The picture provides information on the stimulation of neurogenesis in old rats by the induction of neuroinflammation and the introduction of the fractal protein. These data demonstrate the fact that the brain retains the adaptive function of neurogenesis.
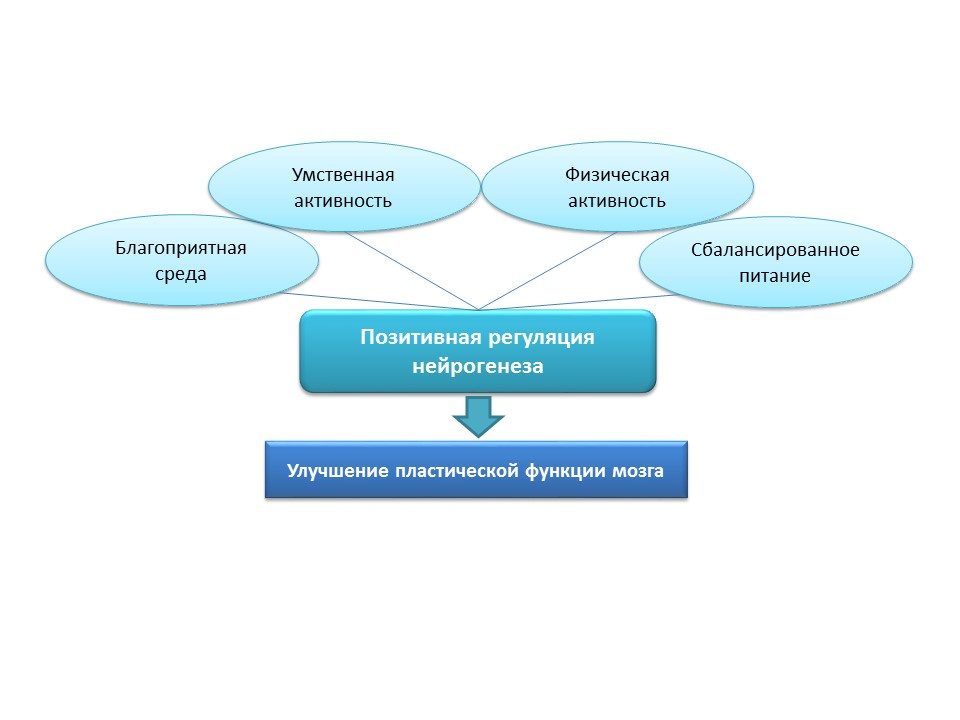
For natural stimulation of neurogenesis in the elderly in humans, these strategies seem to be overly aggressive, so natural positive stimuli should be considered as “training” and “supporting” neurogenesis factors: favorable environment, mental activity, physical activity, balanced nutrition.
Medvedeva, EV, Dmitrieva, VG, Stavchansky, VV et al. Int J Pept Res Ther (2016) 22: 197.
Jakubs K et al. Inflammation regulates the functional integration of neurons born in adult brain. J Neurosci. 2008 Nov 19; 28 (47): 12477-88
Taupin P. Nootropic agents stimulate neurogenesis. Expert Opin Ther Pat. 2009 May;19(5):727-30.
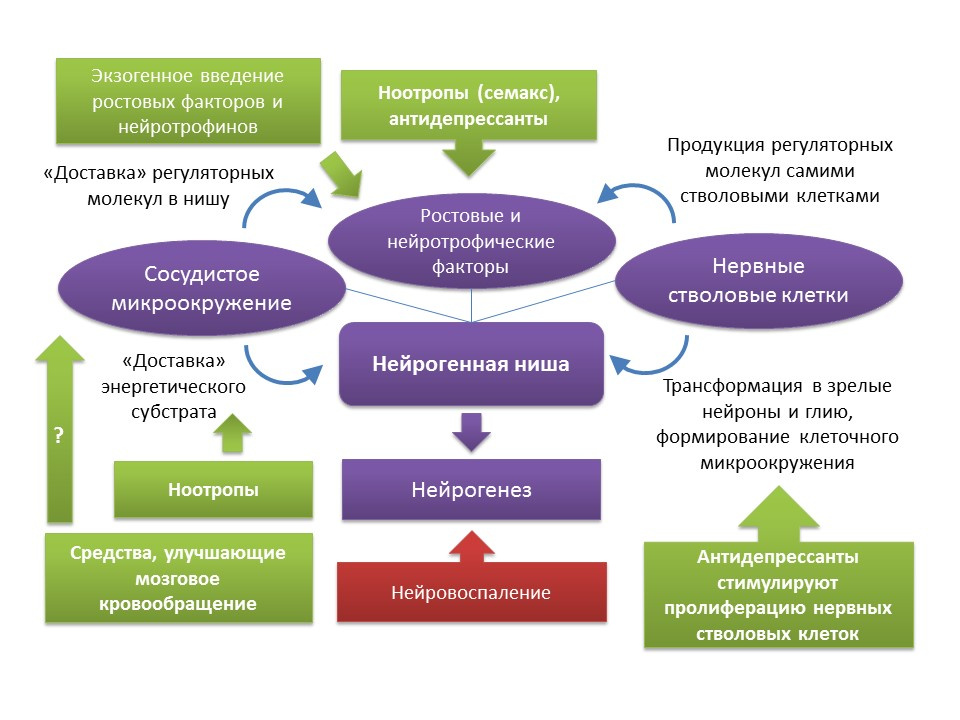
As mentioned earlier, in spite of the age-related changes that a neurogenic niche undergoes, the function of neurogenesis can be restored with certain stimuli. It should be noted that the rates of aging and the age-related changes of neurogenesis associated with this process are heterogeneous and are determined by the individual characteristics of the organism. In particular, it is believed that in individuals with initially higher levels of neurogenesis, they have a higher level of brain neuroplasticity and, therefore, are more resistant to age-related changes in the CNS. However, there are currently no methods for complex effects on the entire neurogenic niche. As mentioned earlier, the neurogenic niche itself is a kind of microenvironment that includes many elements (vascular and cellular microenvironment, a set of growth and neurotrophic factors). One side,There is a fairly solid pharmacological experience in the regulation of neurogenesis due to the impact on the individual components of this “microenvironment” (introduction or induction of endogenous regulatory molecules; stimulation of proliferation, differentiation and survival of newly formed neurons with drugs; improvement of cerebral circulation and energy metabolism in the central nervous system). On the other hand, a well-known fact is the development of growth and neurotrophic factors by the stem nerve cells themselves - BDNF, NGF, VEGF, etc. (a well-known fact is the development of growth and neurotrophic factors by the stem nerve cells themselves - BDNF, NGF, VEGF, etc. (a well-known fact is the development of growth and neurotrophic factors by the stem nerve cells themselves - BDNF, NGF, VEGF, etc. (Guzman R. Cellular stroke therapy: from cell replacement to trophic support. Expert Review of Cardiovascular Therapy.2009;7(10):1187-1190). From this, it can be concluded that, for restoring the function of a neurogenic niche, it is possible to use, for example, therapy by stem cells themselves or by means that are capable of stimulating the proliferation of neural stem cells. To such means, as already mentioned, are antidepressants of various pharmacological groups. Indirect evidence of such a conclusion may be clinical evidence of a reduction in the risk of senile dementia after a course (frequent complications after an earlier depression) antidepressant treatment. It should be noted that data on the effect of antidepressants on the risk of dementia are contradictory. Thus, it has been established that the use of tricyclic antidepressants is associated with a reduced risk of developing dementia, while MAO (monoamine oxidase) inhibitors,heterocyclic antidepressants and SSRIs (selective serotonin reuptake inhibitors), on the contrary, may increase the risk or not have any effect on the development of dementia (Kessing LV, Forman JL, Andersen PK. Do you keep patients with severe depressive disorder? Int Clin Psychopharmacol. 2011; 26 (6): 316-22; Lee CW et al. Antidepressant treatment and risk of dementia: a population-based, retrospective case-control study. J Clin Psychiatry. 2016 Jan; 77 (1): 117-22; quiz 122 ). However, these clinical data require caution when interpreted, since these clinical reviews do not take into account a number of factors (smoking history of patients, alcohol consumption, severity and forms of depressive disorders, comorbidities, etc.).
Another interesting group of drugs that can be considered as potential neurogenic agents are nootropics. Nootropic drugs have a whole number of specific features: 1) they act only against the background of pathology of mnestic functions; 2) have the cumulative nature of the action; 3) diverse in mechanisms of action and chemical structure; 4) act like psychostimulants, but do not have their inherent side effects; 5) today there is no convincing data on the clinical efficacy of nootropics. It is believed that nootropic drugs have a complex effect on the central nervous system: improve cerebral circulation, energy metabolism, interact with the receptors of most neurotransmitters (GABA, glutamate, acetylcholine).Experimental studies have shown the potential ability of some nootropics to stimulate neurogenesis. In particular, piracetam strengthened the process of differentiation in the culture of human neural stem cells (Taupin P. Nootropic agents to stimulate neurogenesis. Expert Opin Ther Pat. 2009 May; 19 (5): 727-30 ), another nootropic drug, Semax, enhanced gene expression of more than 20 growth and neurotrophic factors in the rat brain after intraperitoneal administration under conditions of modeling ischemic stroke ( Medvedeva, EV, Dmitrieva, VG, Stavchansky, VV et al. Int J Pept Res Ther (2016) 22: 197 ), which also indicates the potential pro-neurogenic effect of the drug. Due to the poor knowledge of nootropics, it is difficult to talk about a specific mechanism of their neurogenic activity, in which both their complex effect on the central nervous system and a particular component can take part. Obviously, this issue requires further more detailed studies.
Another group of drugs that could stimulate neurogenesis, are correctors of cerebral circulation (for example, nimodipine or pentoxifylline). However, the data on the effect of these drugs on neurogenesis is quite scarce. Experiments on rats have shown that treatment with pentoxifylline improved short-term memory and decreased apoptosis of hippocampal neurons under conditions of cerebral ischemia, which may indicate its neuroprotective properties ( Park JH et al. Pentoxifylline Alleviates Perinatal Hypoxic-Ischemia-Induced Short-term-for-term-term-term-term-immuno-chemical immunoassay) by Suppressing Apoptosis in the Hippocampus of Rat Pups. Int Neurourol J 2016; 20 (2): 107-113). Nimodipine, in contrast, showed the ability to suppress ischemic-stimulated neurogenesis in the hippocampus of mice (Luo CX et al. Blockage of L-type voltage-gated Ca channel inhibits ischemia-induced neurogenesis by the downward-regulating iNOS expression in adult mouse. Journal of neurochemistry, 2005) that, apparently, is connected with its direct mechanism of action - blockade of L-type calcium channels.
To obtain a complete picture of the pharmacological manipulation of the neurogenic niche in the face of age-related changes, more detailed studies are needed that will broaden the understanding of the activity of neurogenic drugs in relevant conditions and lead to the conclusion of promising directions in the creation of drugs that would prevent and correct the age-related decline in neurogenic brain activity.
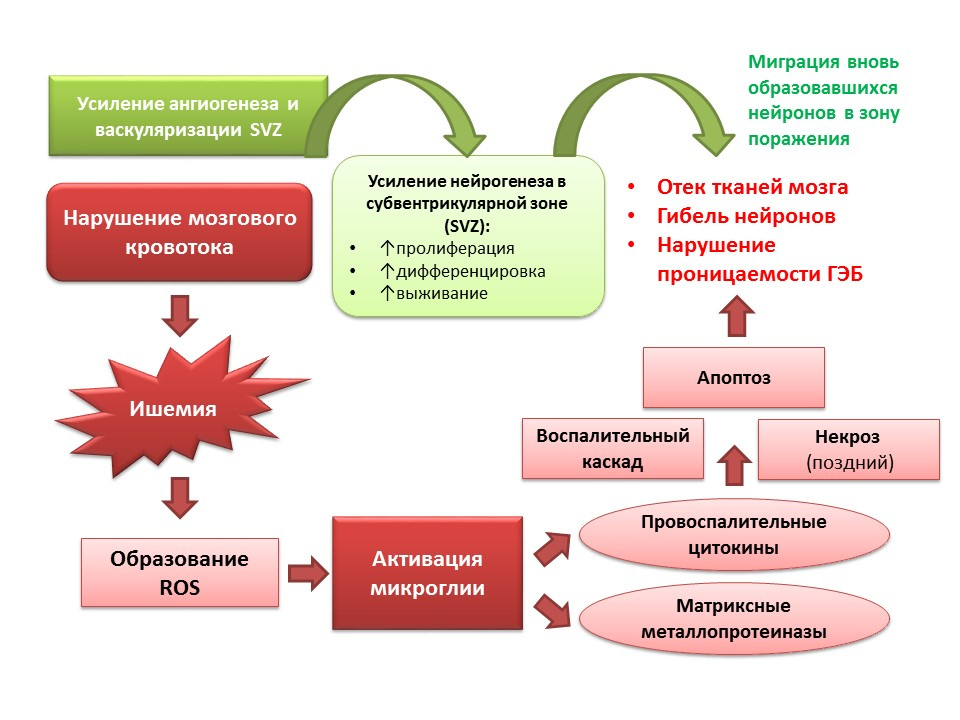
Neurogenesis performs an adaptive function against the background of CNS pathologies. In particular, ischemic stroke leads to the death of nerve cells (a simplified diagram of the pathogenesis is presented on the slide). However, during ischemia, a small number of neurons die quickly (minutes) - this is the so-called core of ischemia. Most of the neurons are deficient in glucose and oxygen, but they remain viable for several hours (the therapeutic window for helping) - this is the so-called penumbra or penumbra ischemia zone. Under conditions of ischemic brain damage, neurogenesis is activated in the subventricular zone of the lateral ventricles and the newly formed neurons migrate to the penumbra zone.
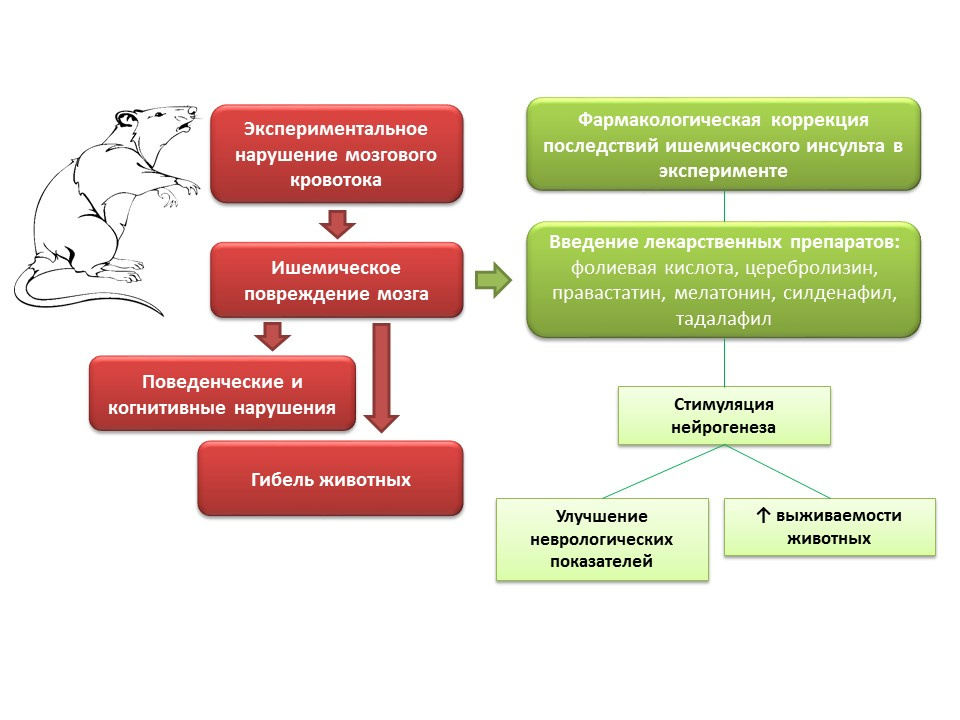
In animal experiments, in modeling of cerebral blood flow disorders, neurological disorders (cognitive and behavioral deficiencies), as well as a large percentage of animal mortality, are observed. Introduction to such animals of drugs with different mechanisms of action, but a common feature - the ability to stimulate neurogenesis (folic acid, cerebrolysin, pravastatin, melatonin, sildenafil, tadalafil) - improving the neurological indicators of animals and significantly reduces mortality.
What are the prospects for the manipulation of neurogenesis in ischemic brain damage?
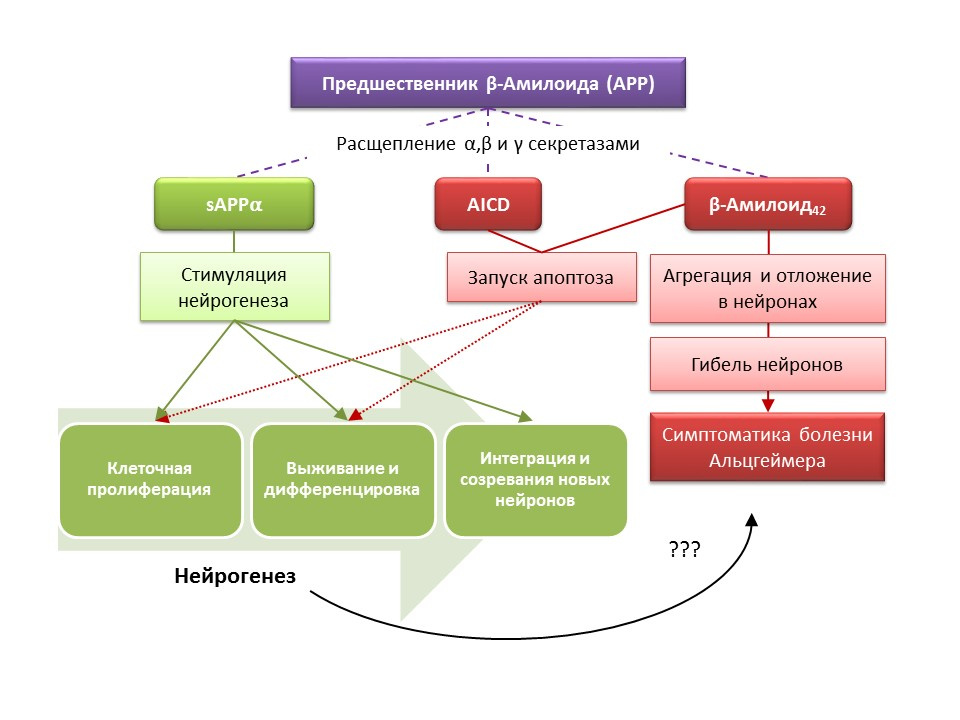
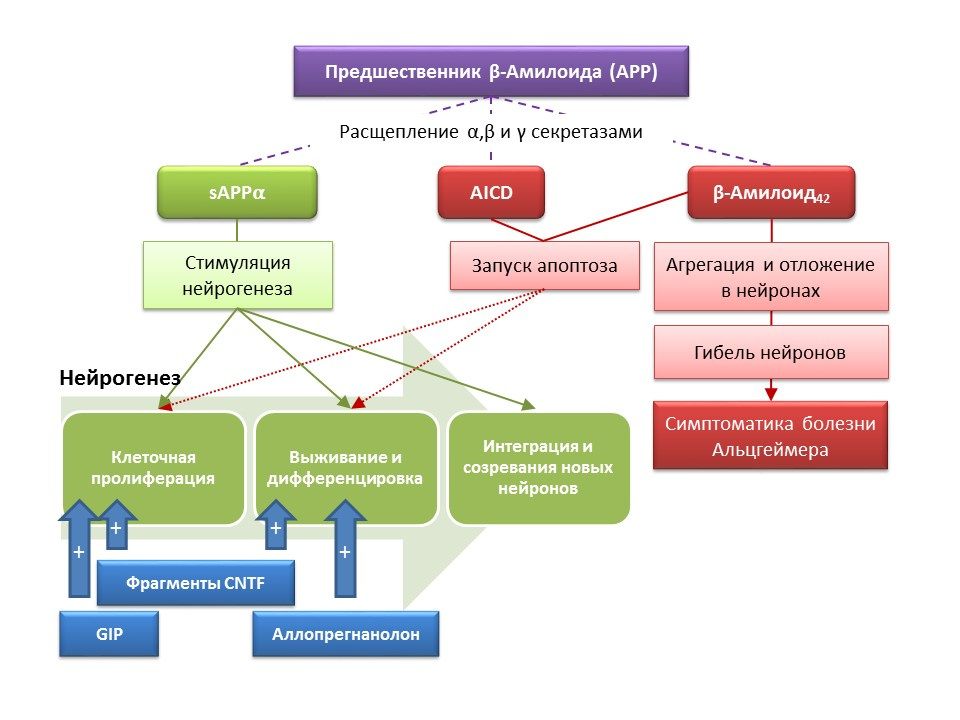
Alzheimer's disease (BA) is a neurodegenerative disease, accompanied by the death of cholinergic neurons (neurons that produce acetylcholine). The causes of Alzheimer's disease are currently not well established, the disease is considered multifactorial. The basis of the pathogenesis of BA is a violation of the metabolism of beta-amyloid protein, separate fractions of which are deposited in neurons of the central nervous system and lead to their death. Amyloid fractions have different effects on neurogenesis, so soluble amyloid precursor alpha (sAPPα) stimulates all stages of neurogenesis, while the intracellular domain of amyloid precursor (AICD) and beta amyloid-42 cause apoptosis of nerve stem cells, reducing proliferation and survival. Activation of neurogenesis is registered already in the early stages of AD, when there is no pronounced neurodegeneration and dementia. Apparently, neurogenesis on the background of BA also plays an adaptive function. On most animal models of BA (transgenic mice with impaired beta-amyloid metabolism) and the postmortem material of patients with BA, an increase in the number of BrdU-positive cells was recorded. However, there is evidence of the absence of changes in neurogenesis in some animal models of BA and the post-mortem material of some patients with BA. Finally, it remains an open question about the very ability of newly formed cells to replace neurons that have died in asthma.
DOI: 10.3233 / JAD-2011-110914
DOI: 10.1016 / j.ejphar.2011.11.117
DOI: 10.1371 / journal.pone.0024293
Experimental studies on new approaches to the pharmacological correction of Alzheimer's disease have demonstrated the ability of a number of substances to stimulate neurogenesis in transgenic mice (in mice, the normal metabolism of beta-amyloid is violated — an animal model of Alzheimer's disease), combined with an improvement in behavioral responses (orienting-research reaction, training and memory):
The results indicate the prospects for the use of substances that stimulate neurogenesis to alleviate the symptoms of Alzheimer's disease, as well as reduce the rate of progression of neurodegeneration, which is a fundamentally new approach to the medical correction of this disease.
Prospects for the manipulation of neurogenesis in Alzheimer's disease
DOI: 10.1016 / j.pharma.2013.02.006
PMID: 11124987
DOI: 10.1186 / s13045-017-0499-7
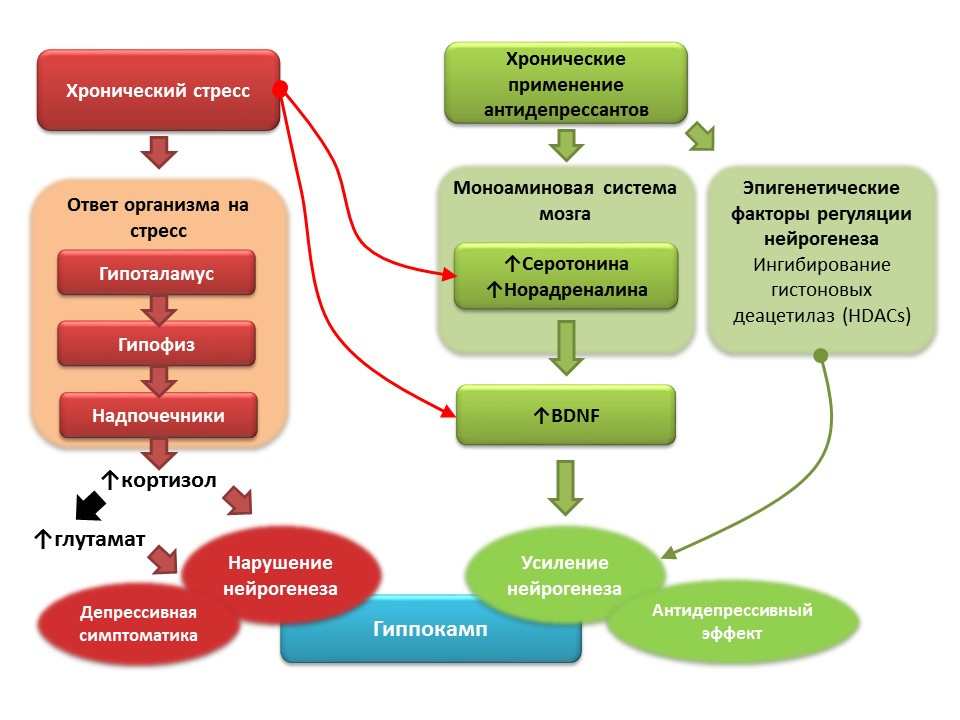
Against the background of clinical depression (major depressive disorder), a decrease in neurogenesis and a decrease in the volume of the hippocampus, which is restored after a course of antidepressants, are also recorded. It is believed that, based on the pathogenesis of depressive disorders, the role of starting factor is played by chronic stress, which triggers a cascade of stress-dependent reactions, culminating in hyperproduction of cortisol and an increase in glutamate concentration in the central nervous system. These factors violate the plasticity of the brain (and neurogenesis in particular) and contribute to the development of depressive symptoms). Chronic use of antidepressants, in contrast, stimulates neurogenesis, increasing the concentration in the CNS of monoamines (serotonin and norepinephrine), a brain neurotrophic factor. Some antidepressants have additional mechanisms for the regulation of neurogenesis. For example, amitriptyline is able to inhibit histone deacetylases, which play the role of epigenetic regulators of neurogenesis, and thereby stimulates the proliferation of nerve stem cells.
Prospects for the manipulation of neurogenesis in depression
General perspectives and approaches to the study of neurogenesis
1. The study of the molecular mechanisms of regulation of neurogenesis in the mature brain.
2. Further studies should answer the question: to what extent can newly-formed neurons replace the lost CNS in the course of a specific pathology?
3. It is necessary to evaluate the possible effects of long-term exogenous stimulation of adult neurogenesis.
4. It is necessary to develop a comprehensive strategy for the correction of disorders of neurogenesis, which would take into account all the main factors:

To positive incentives, i.e. stimuli that contribute to the process of neurogenesis include:
- learning process,
- ecological environment (favorable environment),
- exercise (for example, running),
- antidepressants
- estrogens, etc.
To negative -
- stress,
- excessive glutamate activity in the central nervous system,
- effects of glucorticoids (cortisol is a stress hormone),
- aging and others.
For many years, central dogmas existed in neuroscience, which did not allow the very possibility of neurogenesis.

The ideas about the absence of neurogenesis in the brain of mature vertebrates were based on four principles:
- Clinical. Patients suffering from neurological pathologies with a primary lesion of the central nervous system, do not experience functional recovery. Cerebrovascular disorders, traumatic injuries and neurodegenerative diseases, such as Parkinson's and Alzheimer's disease, are pathologies of the central nervous system with progressive and worsening of the patient's clinical condition. In general, various treatment methods can only eliminate the symptoms, but not stop the development of the disease.
- Functional. It is based on the fact that the central nervous system controls many complex processes: the regulation of emotions, movements, reflexes, etc. The management of such complex processes requires extremely precise and fine "settings". It was believed that the formation of new nerve cells, their differentiation and migration could disrupt the structural and functional organization of existing neural pathways and disrupt the work of the central nervous system.
- Associated with the theory of memory and learning. For a long time, it was thought that “memories” are the extraction of information from neural networks formed in the learning process. In this context, the formation of new nerve cells was considered as an event incompatible with memory.
- Technical and experimental. Lack of technical capacity for the discovery of neurogenesis and neural stem cells in the mature CNS.
Stages of the formation of ideas about the neurogenesis of the mature brain
- 60s - Joseph Altman et al. Cells of the dentate gyrus of the hippocampus can include radioactive thymidine, which is inserted into the DNA of dividing cells, allowing them to be visualized. Due to the lack of molecular markers, it was impossible to prove that it was neurons that were labeled.
DOI: 10.1126 / science.135.3509.1127
- 70s - Michael Kaplan and James Hinds. Reproduced the experience of Altman and using electron microscopy confirmed that it is labeled neurons (dentate gyrus and olfactory bulb).
DOI: 10.1126 / science.887941
- The 80s - Fernando Nottebome. He showed that in the mating season in the brain nuclei of canaries responsible for vocalization and learning, the number of neurons sharply increases.
DOI: 10.1038 / scientificamerican0289-74
- 90s - Peter Ericsson and co-authors. For the first time showed the formation of new neurons in the human hippocampus.
DOI: 10.1038 / 3305
The main neurogenic zones of the adult (or mature) brain are the dentate gyrus of the hippocampus and the subventricular zone of the lateral ventricles. Zones are shown.
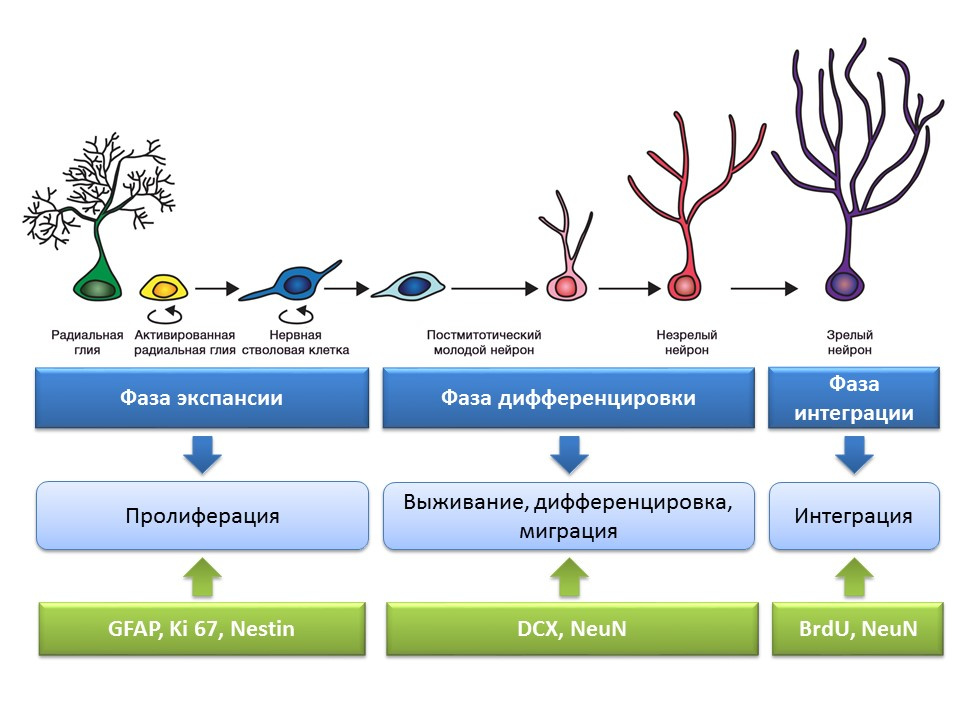
Stages of neurogenesis in the dentate gyrus of the hippocampus of the mature brain
Proliferation - the active process of self-renewal, the possibility of increasing the pool of stem cells.
Differentiation - transformation into a neuron with a specific function, size, metabolism. In fact, the "career guidance" of future nerve cells.
Survival is the selection of newly formed neurons, after which they migrate and integrate into the neural network. The selection of nerve cells is apoptosis (programmed death) of the part of the formed neurons. The remaining cells migrate to the appropriate area of the brain and integrate into the neural network.
The term “neurogenic niche” is inseparably connected with the term “neurogenesis”. By itself, "neurogenic niche" is a "microsphere" in which the process of neurogenesis itself takes place. Neurogenic niche includes:
- vascular microenvironment, which, firstly, supplies blood to the niche itself and ensures delivery of various regulatory molecules (stem cells are formed and transformed in close proximity to blood vessels, secondly, the vascular endothelium growth factor (VEGF) is an important regulatory factor not only in the process of angiogenesis (the growth of new vessels in the existing vascular system) and vasculogenesis (formation of the embryonic vascular system), but also a direct regulator of neurogenesis;
- cellular microenvironment, which includes the formation of nerve cells at different stages of development and glial cells that perform trophic function (produce growth factors, for example, brain neurotrophic factor - BDNF and nerve growth factor - NGF);
- expression in the niche of growth and regulatory factors. In addition to glial cells and other mature niche structures, stem cells are capable of producing regulatory and growth factors, thus performing self-regulation (autocrine regulation).
Evaluation of the processes of neurogenesis using specific markers
An important and interesting issue that deserves special attention is the question of how to detect neurogenesis in the brain tissues of mature mammals.
Bromodeoxyuridine (BrdU) is a thymidine structural analogue - a component of the DNA molecule. When introduced into the body, BrdU is inserted into the DNA of dividing cells instead of thymidine, providing an opportunity to detect the newly formed cells and separate them from the “old” ones. After receiving a sample of brain tissue, they are treated with antibodies to BrdU (antibodies contain a fluorescent label), which bind to BrdU via the mechanism of the immunochemical “antigen-antibody” reaction and provide an opportunity for the colorimetric determination of BrdU. Thus, it is possible to quantify BrdU-labeled cells, so-called BrdU positive cells, on the microslide.
Doublecortin is a protein that is almost always found in immature neurons and allows them to be detected.

A summary diagram of the main methods for detecting the various stages of neurogenesis, which summarizes the presented early information.
Regulation of neurogenesis in the mature brain
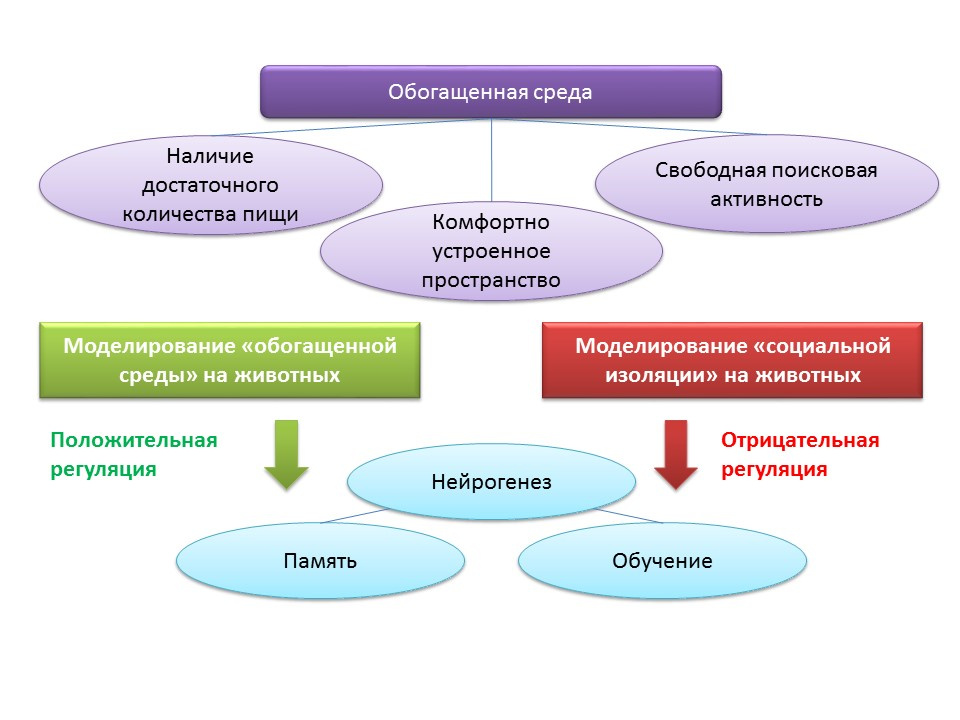
The concept of an “enriched environment” is associated with environmental factors regulating the processes of neurogenesis. The term “enriched environment” itself includes a favorable habitat, namely the presence of a sufficient amount of food, a comfortably arranged space and the possibility for free search activity.
DOI: 10.1002 / hipo.22218
DOI: 10.1038 / 386493a0
DOI: 10.1016 / j.brainres.2011.08.007
DOI: 10.1016 / j.neuroscience.2011.10.040
PMID: 9547229
In animal experiments, it was found that being in an “enriched environment” has a positive effect on neurogenesis: the production of growth factors and neurotrophins, the number of proliferating cells and their survival increase. Increased neurogenesis is correlated with improved cognitive function in animals (mainly with learning and memory processes).
Experimental C57BL / 6J mice for 2 months were kept in a protected area under the conditions of a biological station (in a forest glade), the control group was kept under standard laboratory conditions. In animals of the experimental group, an increase in the number of excitatory and inhibitory synapses was observed compared with the control group. This experiment shows that environmental conditions can favorably influence the morphology and function of the CNS of rodents placed in an enriched environment.
The maintenance of animals in conditions of social isolation in the laboratory, by contrast, acted as a negative regulator of neurogenesis.
Moreover, placing animals in an “enriched environment” after cerebral ischemia contributed to enhancing the regenerative processes in the neurogenic zones of the brain.
The results of such studies are not something extraordinary, since modern medicine uses rehabilitation courses and recreational rest.
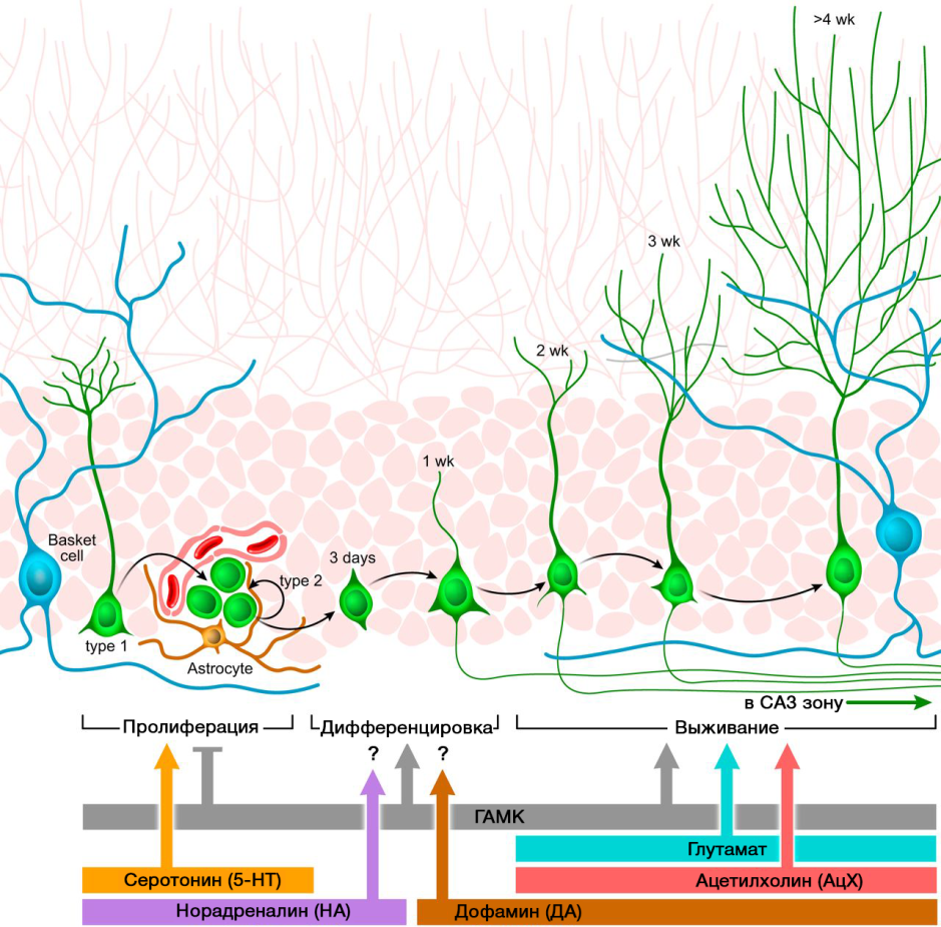
An important role in the regulation of neurogenesis is played by the neurotransmitters of the CNS. The picture shows a summary of such a regulation.

DOI: 10.1152 / physrev.00004.2014
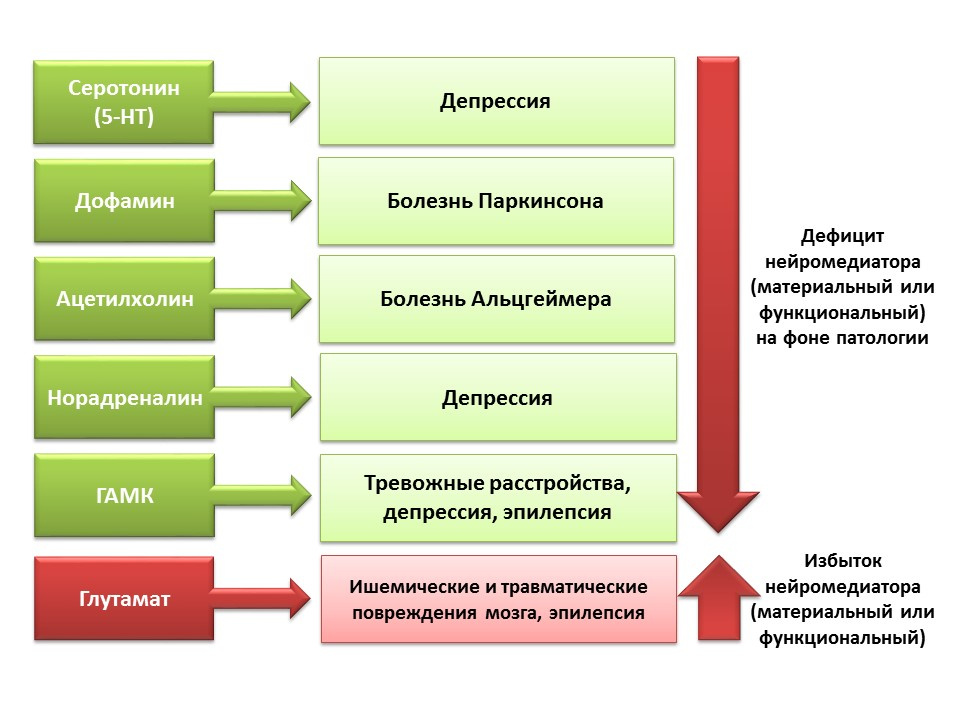
The regulatory role of the presented CNS neurotransmitters in the processes of neurogenesis correlates with a deficiency or excess of these molecules in various diseases of the CNS associated with changes in the level of neurogenesis.
Due to the possibility of pharmacological manipulation of the neurotransmitter system of the CNS, we can evaluate the contribution of a neurotransmitter to different stages of neurogenesis of the mature brain.
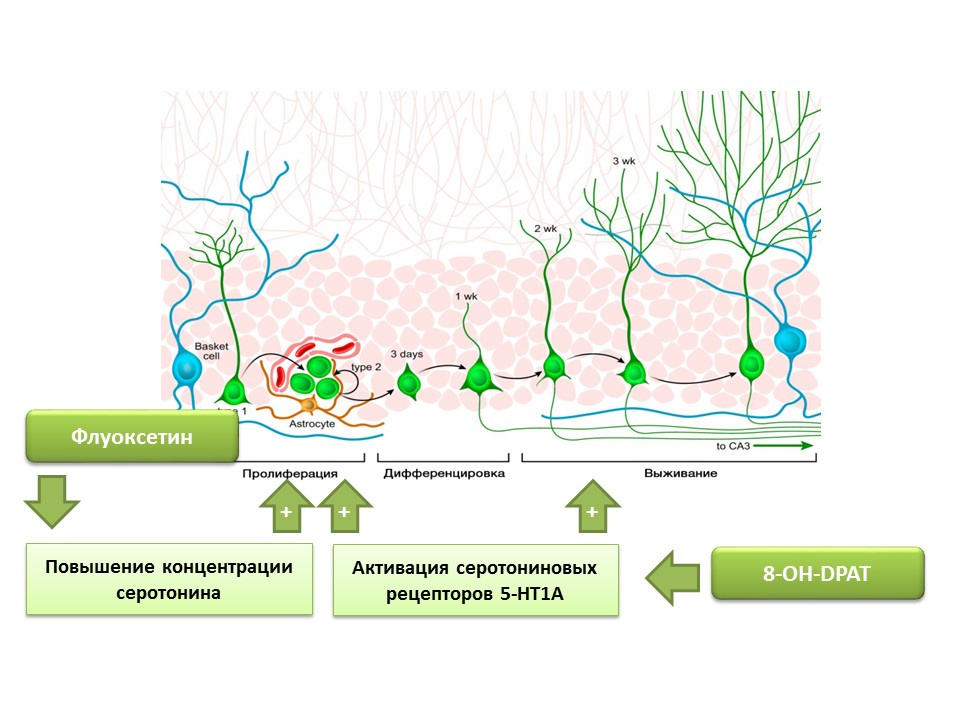
Fluoxetine (an antidepressant from the group of selective serotonin reuptake inhibitors) that can increase the concentration of serotonin in the CNS has been found to increase the proliferation of nerve stem cells in the dentate gyrus of the hippocampus of rodents and primates. Later, on the stem cells themselves, serotonin receptors 1A of the subtype (5-HT1A receptors) were detected and this is consistent with the ability of 8-OH-DPAT (selective activator of these receptors) to stimulate the proliferation and survival of new neurons in the dentate gyrus of experimental animals (mice and rats).
Primates were exposed to chronic stress (social isolation) and then evaluated for depressive-like and anxious behavior, as well as hippocampal neurogenesis (post-mortem). Chronic stress led to a decrease in neurogenesis in combination with behavioral deficits (increased depressive-like and anxious behavior). Treatment with fluoxetine (an antidepressant that increases serotonin concentration in the CNS by suppressing its reuptake) stimulated neurogenesis and prevented depressive-like and anxious behavior.
DOI: 10.1371 / journal.pone.0017600
Another experiment confirms the role of serotonin in the regulation of adult neurogenesis. Chronic stimulation of serotonin receptors 5-1 using substance 8-OH-DPAT in rats resulted in increased proliferation of neural stem cells and survival of differentiating neurons in the dentate gyrus of the hippocampus and the subventricular zone, as well as increased glyogenesis.
DOI: 10.1016 / j.euroneuro.2009.11.007
The presented experiments confirm the important regulatory role of serotonin in the processes of neurogenesis in the adult brain.
DOI: 10.4161 / cc.8.18.9512
DOI: 10.1016 / j.neuropharm.2011.01.026
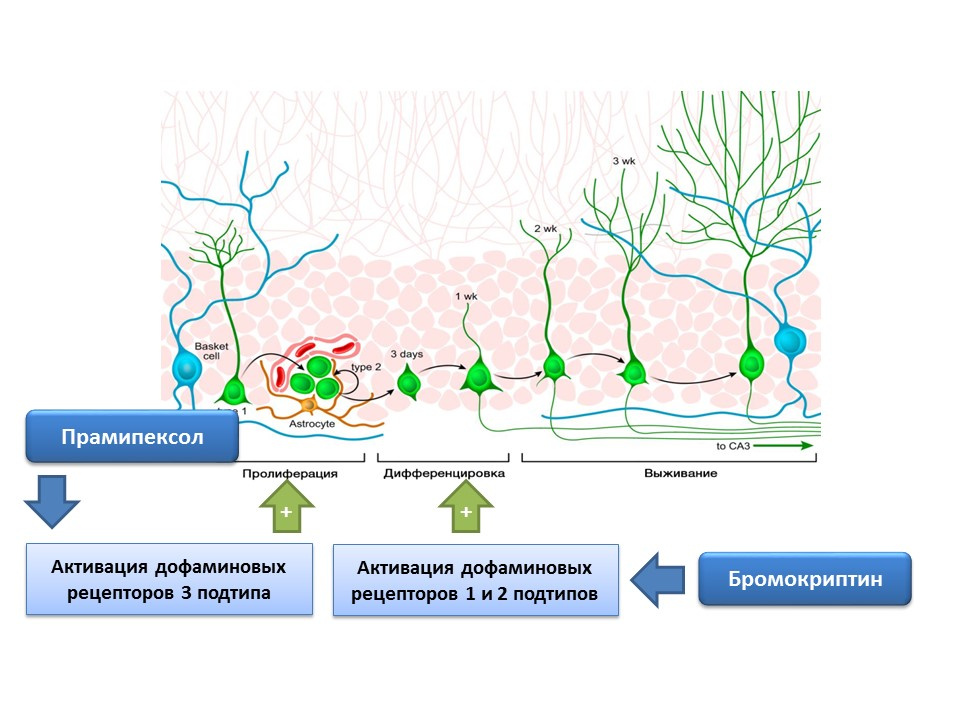
Dopamine is also actively involved in the regulation of neurogenesis. It was shown that activation of dopamine receptors 1 and 2 of type (D1 and D2) by bromocriptine leads to an increase in the differentiation process of neural stem cells, while the stimulation of D3 receptors by pramipexol leads to an increase in proliferation.
Pramipexol is a dopamine receptor agonist, which has a greater affinity for D3 receptors than D2, is used in Parkinson's disease, effectively eliminating the symptoms of this disease. In addition, the drug has neuroprotective activity. In the experiment, the effect of pramipexol on neurogenesis in the cell culture of mice was evaluated. It was shown that the treatment of cell culture with pramipexol led to an increase in the size of neurospheres (clusters of newly formed stem cells) and cells containing doublecortin (immature neurons). The stimulating effect of pramipexole on neurogenesis was eliminated by dopamine receptor blockers - U99194A and sulpiride. In addition, pramipexol has been shown to enhance the release of BDNF. The presented results suggest that the effectiveness of pramipexole in Parkinson's disease is associated not only with the completion of the functional dopamine deficiency, but also with a stimulating effect on neurogenesis, and also indicate the important regulatory role of dopamine in the proliferation of nerve stem cells.
DOI: 10.1016 / j.neuropharm.2011.01.026
The presence of dopamine receptors 1 and 2 subtypes on nerve stem cells was experimentally confirmed, and the important regulatory role of dopamine in proliferation and differentiation on the culture of nerve cells was shown. In particular, the treatment of the cell culture of the subventricular zone with bromocriptine (stimulator of dopamine receptors D1 and D2 subtypes) under conditions of damage by the neurotoxin MPTP (modeling of Parkinson's disease on cell culture) led to an increase in cell proliferation and differentiation.
DOI: 10.4161 / cc.8.18.9512
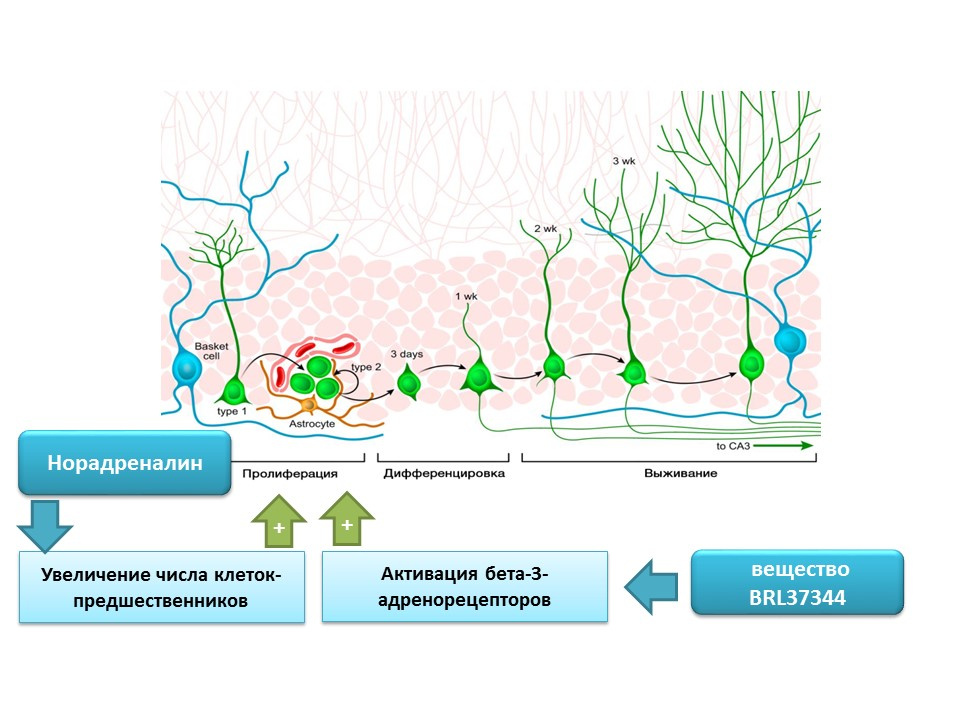
Norepinephrine is able to increase the number of precursors of neural stem cells through the activation of beta-3 adrenoreceptors. This effect was shown using a selective agonist (activator) of beta-3-adrenergic receptors - the substance BRL37344.
The regulatory role of norepinephrine in processes of mature neurogenesis was evaluated in a comprehensive in vitro study (cell culture) in vivo (in mice). Adding norepinephrine to cell culture resulted in an increase in the size of neurospheres (clusters of neural stem cells). Systemic administration of a selective beta-3 adrenoreceptor agonist resulted in increased proliferation of neural stem cells in the hippocampus of mice. These studies confirm data on the presence of adrenoreceptors on immature nerve cells, and also indicate the regulatory role of norepinephrine, which, apparently, is realized through beta-3-adrenoreceptors.
DOI: 10.1523 / JNEUROSCI.3780-09.2010
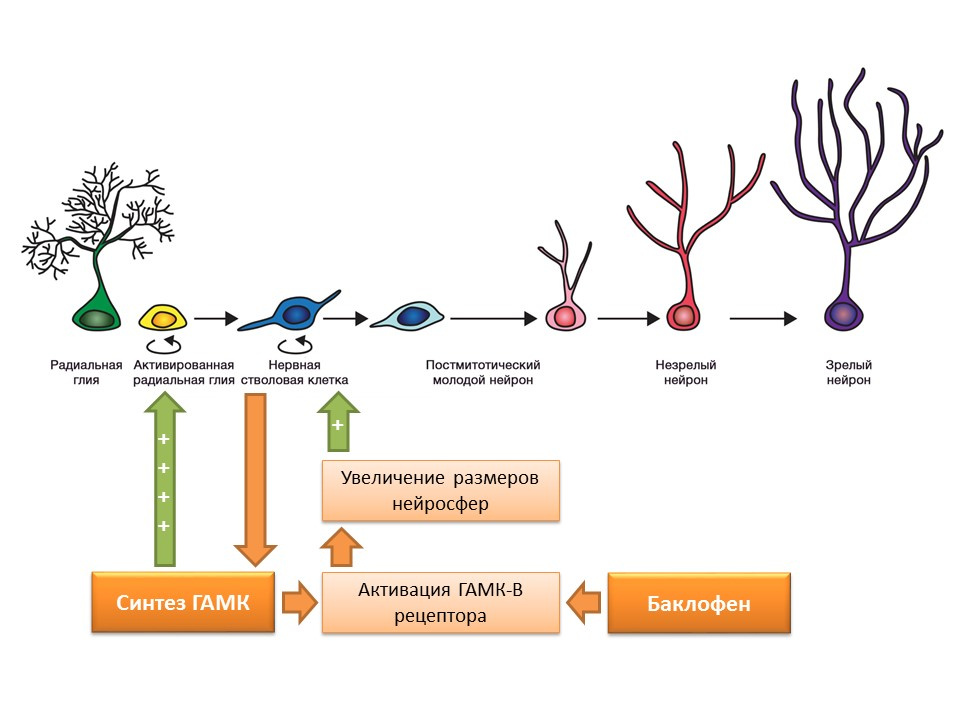
Nerve stem cells, as mentioned earlier, are capable of producing regulation factors themselves. One of these factors is gamma-aminobutyric acid (GABA) - the main inhibitory mediator of the central nervous system. In particular, in the culture of mouse neural cell progenitors, it was shown that neural stem cells enhance GABA synthesis, which in turn enhances proliferation, activating radial glia (gives rise to stem cells) and promotes an increase in the size of neurospheres (accumulation of stem cells) in the experiment.
It was found that this effect of GABA is realized through GABA-B receptors. In studies, a selective GABA-B receptor agonist, baclofen, was used.
DOI: 10.1002 / jcp.21422
Nakamichi N, Takarada T, Yoneda Y. Neurogenesis mediated by gamma-aminobutyric acid and glutamatesignaling. J. Pharmacol Sci. 2009; 110 (2): 133-49
The role of the main excitatory mediator in the central nervous system is twofold. On the one hand, glutamate inhibits the proliferation of undifferentiated stem cells. On the other hand, it stimulates the differentiation of progenitors into the glial line. It is believed that certain ratios of glutamate and GABA are necessary for regulating the differentiation of neural stem cells into certain types of neurons. For example, glutamate, as already mentioned, promotes the formation of glial cells (astrocytes), and GABA contributes to the formation of neurons that synthesize GABA itself (GABAergic neurons).
Kotani S et al. H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis.Chem Biol Interact. 2008; 175 (1-3): 227-30
Kita Y et al. Galantamine promotes adult hippocampal neurogenesis via muscine and α7 nicotinic receptors in mice. Int J Neuropsychopharmacol.2014; 17 (12): 1957-68.
The regulatory role of acetylcholine (ACh) is shown in rats with substances that prevent its destruction (acetylcholinesterase inhibitors - an enzyme that cleaves Ac5). Donepezil and galantamine increase the concentration of ACh in the CNS (donepezil also increases the expression of the CREB protein - a transcription factor, thereby enhancing the protection of immature neurons from apoptosis) and increasing the survival of immature neurons. The positive effect of galantamine on neurogenesis is eliminated by scopolamine (a blocker of all muscarinic cholinoreceptor subtypes) and telenzepine (blocker 1 of muscarinic cholinoreceptor subtypes), which suggests the implementation of ACh on neurogenesis through the activation of muscarinic receptors of type 1 ( Kotani S et al. H. inhibitor, enhances adult hippocampal neurogenesis, Chem Biol Interact. 2008; 175 (1-3): 227-30; Int. Neuropsychopharmacol 2014; 17 (12): 1957-68. ).
Sources
Shetty AK. 19 rat hippocampus generate region-specific neuronal phenotypes in vitro. Hippocampus. 2004; 14 (5): 595-614
Gómez-Lira G et al. Programmed and induced phenotype of hippocampal granule cells. J Neurosci. 2005; 25 (30): 6939-46
Nakamichi N, Takarada T, Yoneda Y. Neurogenesis mediated by gamma-aminobutyric acid and glutamate signaling. J. Pharmacol Sci. 2009; 110 (2): 133-49
Arenas E. Engineering a dopaminergic phenotype in stem / precursor cells: glia-derived signals, and Wnts. Ann NY Acad Sci. 2005; 1049: 51-66
Gómez-Lira G et al. Programmed and induced phenotype of hippocampal granule cells. J Neurosci. 2005; 25 (30): 6939-46
Nakamichi N, Takarada T, Yoneda Y. Neurogenesis mediated by gamma-aminobutyric acid and glutamate signaling. J. Pharmacol Sci. 2009; 110 (2): 133-49
Arenas E. Engineering a dopaminergic phenotype in stem / precursor cells: glia-derived signals, and Wnts. Ann NY Acad Sci. 2005; 1049: 51-66
Under experimental conditions (in animals or in cell culture), it was shown that changing the ratio of regulatory factors and the addition of neurotransmitters themselves to neural stem cells allows one to “program” the neurotransmitter phenotype (that is, the function of the future cell) of newly formed neurons. For example, the cultivation of stem cells with astrocytes allows you to get a population of glutamatergic neurons (neurons that produce glutamate), and with dopamine - dopaminergic.
It is possible that such a directed effect in the future can be used for the selective formation of nerve cells of a particular phenotype. For example, if we know which type is lost in a particular disease (in Parkinson's disease, dopaminergic die, in Alzheimer's disease, cholinergic). But at present, this aspect of neurogenesis remains unexplored until the end, and it is still too early to talk about programmed regulation of neurogenesis (there are isolated studies).
Sources
Pencea V et al. Infusion of BDNF leads to a rat, a septum, a thalamus, and a hypothalamus. J. Neurosci. 2001; 21: 6706–6717
Bath KG et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008; 28: 2383–2393
Gascon E, Vutskits L, Zhang H, et al. Sequential activation of p75 and TrkB is involved in the dendritic development of subventricular zone-derived neuronal progenitors in vitro. Eur J Neurosci. 2005; 21: 69–80
Kahn MA, Kumar S, Liebl D, et al. Mice lacking NT-3, and its receptor TrkC, exhibit profound deficiencies in CNS glial cells. Glia. 1999; 26: 153–165
Shimazu K, Zhao M, Sakata K, et al. NT-facilitates hippocampal plasticity and learning by regulating neurogenesis. Learn Mem. 2006; 13 (3): 307-15)
Zhao M, Li D, Shimazu K, et al. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007; 62: 381–390
Bath KG et al. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008; 28: 2383–2393
Gascon E, Vutskits L, Zhang H, et al. Sequential activation of p75 and TrkB is involved in the dendritic development of subventricular zone-derived neuronal progenitors in vitro. Eur J Neurosci. 2005; 21: 69–80
Kahn MA, Kumar S, Liebl D, et al. Mice lacking NT-3, and its receptor TrkC, exhibit profound deficiencies in CNS glial cells. Glia. 1999; 26: 153–165
Shimazu K, Zhao M, Sakata K, et al. NT-facilitates hippocampal plasticity and learning by regulating neurogenesis. Learn Mem. 2006; 13 (3): 307-15)
Zhao M, Li D, Shimazu K, et al. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007; 62: 381–390
An important role in the regulation of neurogenesis, as mentioned earlier, is played by growth and neurotrophic factors, including:
- brain neurotrophic factor (BDNF)
- nerve growth factor (NGF)
- neurotrophin-3 (NT-3)
- vascular endothelial growth factor (VEGF)
- platelet growth factor (PGF)
- fibroblast growth factor-2 (FGF-2)
- epidermal growth factor (EGF)
In animal experiments (rats and mice), it was shown that intracerebral administration of these factors contributes to the enhancement of all stages of neurogenesis. Congenital deficiency (transgenic mice with knockout genes of relevant factors) leads to impaired neurogenesis, as well as behavioral and cognitive deficits. These data confirm the important regulatory role of these factors in neurogenesis.
Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci.2010; 13 (11): 1338-44.
For all stages of neurogenesis, epigenetic factors of regulation have been established, for example:
- histone deacetylase (HDACs)
- DNA methyltransferase (DNMTs)
- micro RNA
- other.
Sources
Coras R, Siebzehnrubl FA, Pauli E, et al. Low cells correlate with memory dysfunction in humans. Brain. 2010; 133 (11): 3359-72
Cheyne JE, Grant L, Butler-Munro C, et al. Integration of the newly generated neurons in rat dissociated hippocampal cultures. Mol Cell Neurosci. 2011; 47 (3): 203-14
Hernández-Rabaza V, et al. Neuroscience. 2009; 159 (1): 59-68).
Mochizuki N, Moriyama Y, Takagi N, et al. Intravenous injection of neural progenitor cells improved cerebral ischemia-induced learning dysfunction. Biol Pharm Bull. 2011; 34 (2): 260-5
Sahay A., Hen R. Hippocampal neurogenesis and depression. Novartis Found Symp. 2008; 289: 152-60
Toni N, Laplagne DA, Zhao C, et al. Neurons born in the adult dentate gyrus form of functional synapses with target cells. Nat Neurosci. 2008; 11: 901–7
Cheyne JE, Grant L, Butler-Munro C, et al. Integration of the newly generated neurons in rat dissociated hippocampal cultures. Mol Cell Neurosci. 2011; 47 (3): 203-14
Hernández-Rabaza V, et al. Neuroscience. 2009; 159 (1): 59-68).
Mochizuki N, Moriyama Y, Takagi N, et al. Intravenous injection of neural progenitor cells improved cerebral ischemia-induced learning dysfunction. Biol Pharm Bull. 2011; 34 (2): 260-5
Sahay A., Hen R. Hippocampal neurogenesis and depression. Novartis Found Symp. 2008; 289: 152-60
Toni N, Laplagne DA, Zhao C, et al. Neurons born in the adult dentate gyrus form of functional synapses with target cells. Nat Neurosci. 2008; 11: 901–7
Neurogenesis has an important adaptation function in the central nervous system, which is the formation of new synaptic connections (with the participation of new nerve cells), remodeling (restructuring) of existing neural networks depending on the effects of external factors (training, physical activity, stress, etc.), "Reconstruction" of lost synaptic connections (under the influence of external and internal factors).
All the listed adaptive changes have a direct impact on the emotional response, learning processes and memory. Neurogenesis helps the nervous system to maintain "plasticity", to change and rebuild under the new conditions and objectives.
Sources
Lichtenwalner RJ et al. Neuroscienc 2001; 107, 603–613
Luo J, et al. Aging Cell. 2006; 5 (2): 139-52
Cuppini R et al. Hippocampus. 2006; 16 (2): 141-8;
Shetty AK, Hattiangady B, Shetty GA. Glia 2005; 51, 173–186
Drapeau E, Nora Abrous D. Aging Cell. 2008; 7 (4): 569-89
Hattiangady B, Shetty AK. Neurobiol. Aging.2008; 29: 129–177
Luo J, et al. Aging Cell. 2006; 5 (2): 139-52
Cuppini R et al. Hippocampus. 2006; 16 (2): 141-8;
Shetty AK, Hattiangady B, Shetty GA. Glia 2005; 51, 173–186
Drapeau E, Nora Abrous D. Aging Cell. 2008; 7 (4): 569-89
Hattiangady B, Shetty AK. Neurobiol. Aging.2008; 29: 129–177
With age, the processes of neurogenesis begin to fade. At the molecular level, aging is accompanied by the following changes in neurogenesis:
- lack of growth factors and neurotrophin - one of the main regulators;
- the neurotransmitter brain balance changes (the number of mediators decreases, while others increase the number of mediators);
- decreases the intensity of all phases of neurogenesis;
- blood supply to the neurogenic niche is reduced (angiogenesis is reduced, expression of vascular endothelial growth factor - VEGF is reduced), which reduces the availability of regulatory factors for stem cells;
- the distance between neural stem cells and vascular endothelial cells increases (normally, stem cells are transformed near vessels).
However, age-related changes in neurogenesis are not irreversible, and the adaptive function of the brain can be stimulated.
- In experimental studies on rats, it was shown that neuronal inflammation can promote the integration of newly formed nerve cells into the neural network, i.e. stimulated the final phase of neurogenesis (Jakubs K et al. Inflammation regulates the functional integration of neurons born in an adult brain. J Neurosci. 2008 Nov 19; 28 (47): 12477-88).
- , . . (Bachstetter AD et al. Fractalkine and CX3CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32(11):2030-44).
The picture provides information on the stimulation of neurogenesis in old rats by the induction of neuroinflammation and the introduction of the fractal protein. These data demonstrate the fact that the brain retains the adaptive function of neurogenesis.
For natural stimulation of neurogenesis in the elderly in humans, these strategies seem to be overly aggressive, so natural positive stimuli should be considered as “training” and “supporting” neurogenesis factors: favorable environment, mental activity, physical activity, balanced nutrition.
Medvedeva, EV, Dmitrieva, VG, Stavchansky, VV et al. Int J Pept Res Ther (2016) 22: 197.
Jakubs K et al. Inflammation regulates the functional integration of neurons born in adult brain. J Neurosci. 2008 Nov 19; 28 (47): 12477-88
Taupin P. Nootropic agents stimulate neurogenesis. Expert Opin Ther Pat. 2009 May;19(5):727-30.
As mentioned earlier, in spite of the age-related changes that a neurogenic niche undergoes, the function of neurogenesis can be restored with certain stimuli. It should be noted that the rates of aging and the age-related changes of neurogenesis associated with this process are heterogeneous and are determined by the individual characteristics of the organism. In particular, it is believed that in individuals with initially higher levels of neurogenesis, they have a higher level of brain neuroplasticity and, therefore, are more resistant to age-related changes in the CNS. However, there are currently no methods for complex effects on the entire neurogenic niche. As mentioned earlier, the neurogenic niche itself is a kind of microenvironment that includes many elements (vascular and cellular microenvironment, a set of growth and neurotrophic factors). One side,There is a fairly solid pharmacological experience in the regulation of neurogenesis due to the impact on the individual components of this “microenvironment” (introduction or induction of endogenous regulatory molecules; stimulation of proliferation, differentiation and survival of newly formed neurons with drugs; improvement of cerebral circulation and energy metabolism in the central nervous system). On the other hand, a well-known fact is the development of growth and neurotrophic factors by the stem nerve cells themselves - BDNF, NGF, VEGF, etc. (a well-known fact is the development of growth and neurotrophic factors by the stem nerve cells themselves - BDNF, NGF, VEGF, etc. (a well-known fact is the development of growth and neurotrophic factors by the stem nerve cells themselves - BDNF, NGF, VEGF, etc. (Guzman R. Cellular stroke therapy: from cell replacement to trophic support. Expert Review of Cardiovascular Therapy.2009;7(10):1187-1190). From this, it can be concluded that, for restoring the function of a neurogenic niche, it is possible to use, for example, therapy by stem cells themselves or by means that are capable of stimulating the proliferation of neural stem cells. To such means, as already mentioned, are antidepressants of various pharmacological groups. Indirect evidence of such a conclusion may be clinical evidence of a reduction in the risk of senile dementia after a course (frequent complications after an earlier depression) antidepressant treatment. It should be noted that data on the effect of antidepressants on the risk of dementia are contradictory. Thus, it has been established that the use of tricyclic antidepressants is associated with a reduced risk of developing dementia, while MAO (monoamine oxidase) inhibitors,heterocyclic antidepressants and SSRIs (selective serotonin reuptake inhibitors), on the contrary, may increase the risk or not have any effect on the development of dementia (Kessing LV, Forman JL, Andersen PK. Do you keep patients with severe depressive disorder? Int Clin Psychopharmacol. 2011; 26 (6): 316-22; Lee CW et al. Antidepressant treatment and risk of dementia: a population-based, retrospective case-control study. J Clin Psychiatry. 2016 Jan; 77 (1): 117-22; quiz 122 ). However, these clinical data require caution when interpreted, since these clinical reviews do not take into account a number of factors (smoking history of patients, alcohol consumption, severity and forms of depressive disorders, comorbidities, etc.).
Another interesting group of drugs that can be considered as potential neurogenic agents are nootropics. Nootropic drugs have a whole number of specific features: 1) they act only against the background of pathology of mnestic functions; 2) have the cumulative nature of the action; 3) diverse in mechanisms of action and chemical structure; 4) act like psychostimulants, but do not have their inherent side effects; 5) today there is no convincing data on the clinical efficacy of nootropics. It is believed that nootropic drugs have a complex effect on the central nervous system: improve cerebral circulation, energy metabolism, interact with the receptors of most neurotransmitters (GABA, glutamate, acetylcholine).Experimental studies have shown the potential ability of some nootropics to stimulate neurogenesis. In particular, piracetam strengthened the process of differentiation in the culture of human neural stem cells (Taupin P. Nootropic agents to stimulate neurogenesis. Expert Opin Ther Pat. 2009 May; 19 (5): 727-30 ), another nootropic drug, Semax, enhanced gene expression of more than 20 growth and neurotrophic factors in the rat brain after intraperitoneal administration under conditions of modeling ischemic stroke ( Medvedeva, EV, Dmitrieva, VG, Stavchansky, VV et al. Int J Pept Res Ther (2016) 22: 197 ), which also indicates the potential pro-neurogenic effect of the drug. Due to the poor knowledge of nootropics, it is difficult to talk about a specific mechanism of their neurogenic activity, in which both their complex effect on the central nervous system and a particular component can take part. Obviously, this issue requires further more detailed studies.
Another group of drugs that could stimulate neurogenesis, are correctors of cerebral circulation (for example, nimodipine or pentoxifylline). However, the data on the effect of these drugs on neurogenesis is quite scarce. Experiments on rats have shown that treatment with pentoxifylline improved short-term memory and decreased apoptosis of hippocampal neurons under conditions of cerebral ischemia, which may indicate its neuroprotective properties ( Park JH et al. Pentoxifylline Alleviates Perinatal Hypoxic-Ischemia-Induced Short-term-for-term-term-term-term-immuno-chemical immunoassay) by Suppressing Apoptosis in the Hippocampus of Rat Pups. Int Neurourol J 2016; 20 (2): 107-113). Nimodipine, in contrast, showed the ability to suppress ischemic-stimulated neurogenesis in the hippocampus of mice (Luo CX et al. Blockage of L-type voltage-gated Ca channel inhibits ischemia-induced neurogenesis by the downward-regulating iNOS expression in adult mouse. Journal of neurochemistry, 2005) that, apparently, is connected with its direct mechanism of action - blockade of L-type calcium channels.
To obtain a complete picture of the pharmacological manipulation of the neurogenic niche in the face of age-related changes, more detailed studies are needed that will broaden the understanding of the activity of neurogenic drugs in relevant conditions and lead to the conclusion of promising directions in the creation of drugs that would prevent and correct the age-related decline in neurogenic brain activity.
The role of neurogenesis in various pathologies of the central nervous system
Sources
Bordt, EA, Polster, BM (2014). NADPH Oxidase- and Mitochondria-derived Reactive Oxygen Species in Proinflammatory Microglial Activation: A Bipartisan Affair? Free Radic Biol Med, 34–46
Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18(19):7768-78
Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38
Nakayama D, Matsuyama T, Ishibashi-Ueda H, et al. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. Eur J Neurosci. 2010;31(1):90-8)
Minger SL, Ekonomou A, Carta EM, et al. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2(1):69-74).
Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18(19):7768-78
Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38
Nakayama D, Matsuyama T, Ishibashi-Ueda H, et al. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. Eur J Neurosci. 2010;31(1):90-8)
Minger SL, Ekonomou A, Carta EM, et al. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2(1):69-74).
Neurogenesis performs an adaptive function against the background of CNS pathologies. In particular, ischemic stroke leads to the death of nerve cells (a simplified diagram of the pathogenesis is presented on the slide). However, during ischemia, a small number of neurons die quickly (minutes) - this is the so-called core of ischemia. Most of the neurons are deficient in glucose and oxygen, but they remain viable for several hours (the therapeutic window for helping) - this is the so-called penumbra or penumbra ischemia zone. Under conditions of ischemic brain damage, neurogenesis is activated in the subventricular zone of the lateral ventricles and the newly formed neurons migrate to the penumbra zone.
Sources
Zhang, RL, Zhang, ZG, & Chopp, M. (2013). Targeting nitric oxide in the subacute restorative treatment of ischemic stroke. Expert Opinion on Investigational Drugs, 22(7), 843–851.
Chern CM, Liao JF, Wang YH, Shen YC. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic Biol Med. 2012;52(9):1634-47
Zhang X, Huang G, Liu H, et al. Folic acid enhances Notch signaling, hippocampal neurogenesis, and cognitive function in a rat model of cerebral ischemia. Nutr Neurosci. 2012;15(2):55-61
Zhang C, Chopp M, Cui Y, et al. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J Neurosci Res. 2010 Nov 15;88(15):3275-81
Zhang RL, Zhang Z, Zhang L, et al. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83(7):1213-9
Zheng Z, Chen B. Effects of Pravastatin on neuroprotection and neurogenesis after cerebral ischemia in rats. Neurosci Bull. 2000;23(4):189-97
Chern CM, Liao JF, Wang YH, Shen YC. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic Biol Med. 2012;52(9):1634-47
Zhang X, Huang G, Liu H, et al. Folic acid enhances Notch signaling, hippocampal neurogenesis, and cognitive function in a rat model of cerebral ischemia. Nutr Neurosci. 2012;15(2):55-61
Zhang C, Chopp M, Cui Y, et al. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J Neurosci Res. 2010 Nov 15;88(15):3275-81
Zhang RL, Zhang Z, Zhang L, et al. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83(7):1213-9
Zheng Z, Chen B. Effects of Pravastatin on neuroprotection and neurogenesis after cerebral ischemia in rats. Neurosci Bull. 2000;23(4):189-97
In animal experiments, in modeling of cerebral blood flow disorders, neurological disorders (cognitive and behavioral deficiencies), as well as a large percentage of animal mortality, are observed. Introduction to such animals of drugs with different mechanisms of action, but a common feature - the ability to stimulate neurogenesis (folic acid, cerebrolysin, pravastatin, melatonin, sildenafil, tadalafil) - improving the neurological indicators of animals and significantly reduces mortality.
What are the prospects for the manipulation of neurogenesis in ischemic brain damage?
- The possibility of using individual drugs from the group of neuroprotectors (Cerebrolysin) as part of the complex therapy of acute ischemic stroke to prevent neuron death in the penumbra area.
- For many drugs that have shown the ability to stimulate neurogenesis and improve the neurological condition of animals after the modeling of ischemic stroke, this effect is not the main one, therefore, their clinical use is hampered by the presence of its own effects, which may be undesirable.
- On the other hand, the study of the mechanisms underlying the neuroprotective effects is an important task, since it can provide the basis for the development of fundamentally new highly effective drugs for restorative therapy of ischemic stroke.
- Pharmacological regulation of neurogenesis, against the background of ischemic brain damage, opens up opportunities for creating a new group of drugs that reduce mortality and improve the quality of life of patients after suffering ischemic stroke.
Sources
Yu Y et al. Increased hippocampal neurogenesis in the progressive stage of Alzheimer's disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus. 2009;19(12):1247-53
Ghosal K, Stathopoulos A, Pimplikar SW. APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS One. 2010;5(7):e11866
Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol Neurodegener. 2011;6:85
Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci USA. 2004; 101(1): 343–347
Ghosal K, Stathopoulos A, Pimplikar SW. APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS One. 2010;5(7):e11866
Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol Neurodegener. 2011;6:85
Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci USA. 2004; 101(1): 343–347
Alzheimer's disease (BA) is a neurodegenerative disease, accompanied by the death of cholinergic neurons (neurons that produce acetylcholine). The causes of Alzheimer's disease are currently not well established, the disease is considered multifactorial. The basis of the pathogenesis of BA is a violation of the metabolism of beta-amyloid protein, separate fractions of which are deposited in neurons of the central nervous system and lead to their death. Amyloid fractions have different effects on neurogenesis, so soluble amyloid precursor alpha (sAPPα) stimulates all stages of neurogenesis, while the intracellular domain of amyloid precursor (AICD) and beta amyloid-42 cause apoptosis of nerve stem cells, reducing proliferation and survival. Activation of neurogenesis is registered already in the early stages of AD, when there is no pronounced neurodegeneration and dementia. Apparently, neurogenesis on the background of BA also plays an adaptive function. On most animal models of BA (transgenic mice with impaired beta-amyloid metabolism) and the postmortem material of patients with BA, an increase in the number of BrdU-positive cells was recorded. However, there is evidence of the absence of changes in neurogenesis in some animal models of BA and the post-mortem material of some patients with BA. Finally, it remains an open question about the very ability of newly formed cells to replace neurons that have died in asthma.
DOI: 10.3233 / JAD-2011-110914
DOI: 10.1016 / j.ejphar.2011.11.117
DOI: 10.1371 / journal.pone.0024293
Experimental studies on new approaches to the pharmacological correction of Alzheimer's disease have demonstrated the ability of a number of substances to stimulate neurogenesis in transgenic mice (in mice, the normal metabolism of beta-amyloid is violated — an animal model of Alzheimer's disease), combined with an improvement in behavioral responses (orienting-research reaction, training and memory):
- The introduction of functional fragments of ciliary neurotrophin CNTF - peptides 6 and 6A stimulated the formation of new stem cells and the survival of newly formed neurons in the subventricular zone, the dentate gyrus of the hippocampus and olfactory bulbs (Rockenstein E, Ubhi K, Doppler E, et al.). of CNTF-derived peptides and cerebrolysin in AβPP transgenic mice. J Alzheimers Dis. 2011; 27 (4): 743-52)
- The introduction of a glucose-dependent insulinotropic peptide (GIF), which has the properties of a growth factor, led to increased proliferation of nerve stem cells in the dentate gyrus of the hippocampus (Faivre E, Hamilton A, Hölscher C.) in mice. Eur J Pharmacol. 2012; 674 (2-3): 294-306)
- The chronic and subchronic administration of the neuroactive steroid allopregnanolone contributed to an increase in the survival rate of newly formed neurons (Chen S, Wang JM, Irwin RW, et al. Allopregnanolone promotes regeneration and preclinical model of Alzheimer's disease. PLoS One.2011; 6 ( 8): e24293; Singh C, Liu L, Wang JM, et al. Neurobiol Aging. 2012; 33 (8): 1493-506)
The results indicate the prospects for the use of substances that stimulate neurogenesis to alleviate the symptoms of Alzheimer's disease, as well as reduce the rate of progression of neurodegeneration, which is a fundamentally new approach to the medical correction of this disease.
Prospects for the manipulation of neurogenesis in Alzheimer's disease
- The exact etiology of Alzheimer's disease today remains unknown, which makes it difficult to search for new drugs aimed at the cause of the disease.
- The data obtained in the course of genetic modeling of Alzheimer's disease in animals and the study of post-mortem material of people with asthma are controversial.
- At the same time, in animal models of Alzheimer's disease, it has been shown that increased neurogenesis is noted already in the early stages of the disease (before cognitive impairment is impaired). It is believed that this enhancement can be regarded as an adaptive defense response of the central nervous system.
- In animal models of Alzheimer's disease, it was shown that a number of substances (active fragments of ciliary neutrophin - CNTF, glucose-dependent insulinotropic peptide - GIP, and allopregnanolone) can stimulate neurogenesis and improve the rodent behavior pattern.
- A significant disadvantage of these manipulations is the fact that a violation of neurogenesis, apparently, is not the cause of Alzheimer's disease, but only a symptom. However, the prospects for such therapy may be in slowing the progression of the disease and delaying the development of dementia.
DOI: 10.1016 / j.pharma.2013.02.006
PMID: 11124987
DOI: 10.1186 / s13045-017-0499-7
Against the background of clinical depression (major depressive disorder), a decrease in neurogenesis and a decrease in the volume of the hippocampus, which is restored after a course of antidepressants, are also recorded. It is believed that, based on the pathogenesis of depressive disorders, the role of starting factor is played by chronic stress, which triggers a cascade of stress-dependent reactions, culminating in hyperproduction of cortisol and an increase in glutamate concentration in the central nervous system. These factors violate the plasticity of the brain (and neurogenesis in particular) and contribute to the development of depressive symptoms). Chronic use of antidepressants, in contrast, stimulates neurogenesis, increasing the concentration in the CNS of monoamines (serotonin and norepinephrine), a brain neurotrophic factor. Some antidepressants have additional mechanisms for the regulation of neurogenesis. For example, amitriptyline is able to inhibit histone deacetylases, which play the role of epigenetic regulators of neurogenesis, and thereby stimulates the proliferation of nerve stem cells.
Prospects for the manipulation of neurogenesis in depression
- Most antidepressants, regardless of the group and mechanism of action, are able to stimulate neurogenesis under conditions of depression modeling in animal models.
- In people with depression, the chronic use of antidepressants restores the volume of the hippocampus, a decrease of which is usually noted in depressive disorders.
- The general mechanism of action of all antidepressants is the effect on the monoamine system, in particular, the ability to increase serotonin and noradrenaline concentrations in the CNS.
- Numerous experimental studies have shown the important role of monoamines (serotonin and noradrenaline) in the regulation of hippocampal neurogenesis in the adult brain.
- A promising direction is the further detailed study of the mechanisms of regulation of neurogenesis by serotonin and norepinephrine, understanding of which may help in the development of newer means of pharmacological correction of depression.
- However, not all antidepressants are able to stimulate neurogenesis by themselves, some only restore the original level of neurogenesis, reduced chronic stress.
- Therefore, another important area is the study of the mechanisms of antidepressants that are not associated with the monoamine system. For example, the effect of antidepressants on epigenetic regulators of neurogenesis.
- A separate area is represented by the development of drugs based on brain neurotrophic factor (BDNF) and the decrease in the effect of glutamate on the central nervous system (glutamate blockers for NMDA receptors).
General perspectives and approaches to the study of neurogenesis
1. The study of the molecular mechanisms of regulation of neurogenesis in the mature brain.
- What systems are involved in the regulation of neurogenesis in the adult brain and what disorders lead to inhibition of neurogenesis?
- Consideration of these systems and their individual components as potential targets for drug correction of neurogenesis.
- Search for new "formulas" of drugs that can regulate the processes of neurogenesis in the adult brain.
2. Further studies should answer the question: to what extent can newly-formed neurons replace the lost CNS in the course of a specific pathology?
3. It is necessary to evaluate the possible effects of long-term exogenous stimulation of adult neurogenesis.
4. It is necessary to develop a comprehensive strategy for the correction of disorders of neurogenesis, which would take into account all the main factors:
- The need for angiogenesis
- Neurogenesis itself
- Synaptogenesis
- Axonal remodeling
- Creation of favorable external conditions
All Articles