Diagnose cancer helps positron
The title of the article was not chosen randomly. The NUST MISiS blog has an article “Laser will help diagnose cancer” with a detailed description of the principle of operation of a laser fluorescent microscope, but, in fact, not a word about cancer diagnostics. Quite a while ago, I had a vague idea to write a short review about this method of diagnosing cancer tumors as positron emission tomography (hereinafter referred to as PET). The news about the construction of a center for nuclear medicine and an article on MRI only strengthened in this thought.
The diagnostic method is based on the fact that some substances characteristic of the human metabolism in general and cancer cells in particular are labeled with a radioactive label and then introduced into the human body. This compound is called radiopharmaceutical - RFP. The subsequent detection of decay products allows us to construct a three-dimensional map of the distribution of the label in the body to determine the absorption regions that are uncharacteristic for a healthy person. An important feature of the PET technique is that beta-plus decay is the dominant decay mechanism, i.e. decay with the formation of a positron.
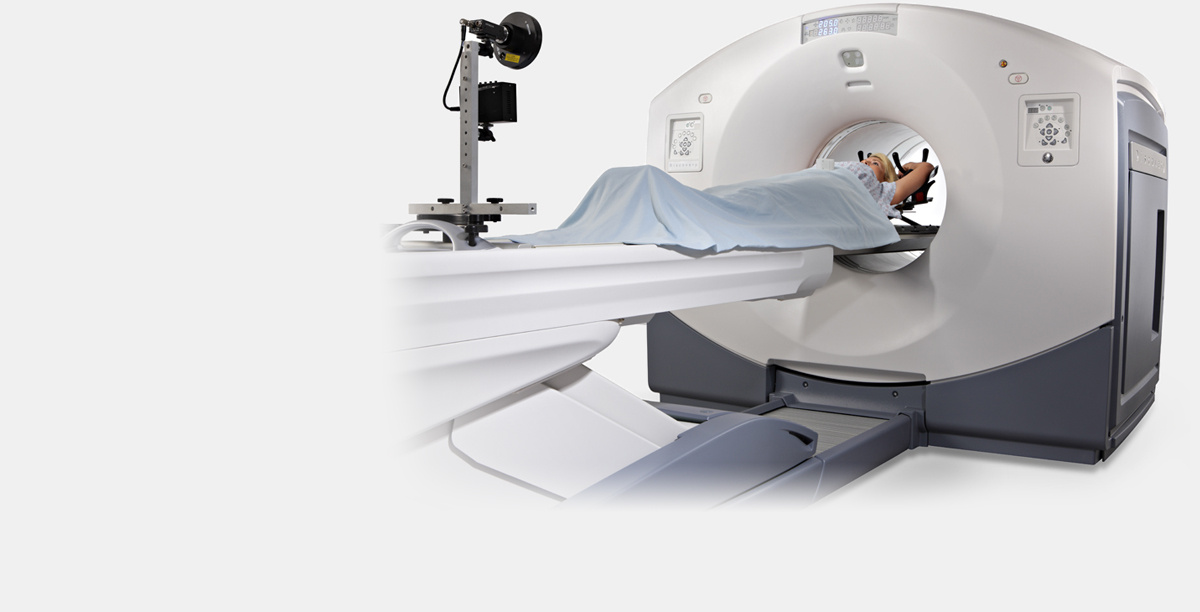
A PET / CT (positron emission combined with a computer) tomograph GE Discovery 610. Image taken from the official website of GE Healthcare. Note The vertical bar at the patient's feet is the breath control system.
Here it is necessary to make a step towards quantum mechanics. Annihilation of a positron and an electron does not occur instantaneously. A positron emitted by a radioactive label, when it encounters an electron, forms a bound state - “positronium”. Both an electron and a positron are fermions, so the total spin of the bound state can be zero (para-positronium) or one (ortho-positronium). The lifetime of para-positronium is about 0.1 ns, whereas ortho-positronium is 3 orders of magnitude longer. Para-positronium can decay only into an even number of gamma-quanta, ortho-positronium, on the contrary, only into an odd number of gamma-quanta. This behavior stems from the laws of conservation of quantum mechanical parities and symmetries. In view of the low positron energies in the case of PET, it can be assumed that only 2-photon and 3-photon decays are possible. In addition, a positron in the composition of ortho-positronium, due to a much longer lifetime, can react with other electrons of the medium with a transition from the ortho-to-para state. In fact, the dominant decay mechanism is the decay with the formation of 2x gamma quanta, although from a quantum-mechanical point of view, the formation of ortho-positronium is 3 times more likely. The above is true only for dense environments, which is the human body. It is important that the emitted gamma-quanta have the same energy of 511 keV and fly away in strictly opposite directions. In the framework of quantum mechanics, this statement can be proved strictly, within the framework of the macro-world mechanics can be represented as follows: while the positronium energy exceeds 1022 keV (the total rest energy of an electron and positron), then positronium "lives and moves", losing energy when interacting with matter. As soon as the positronium energy drops to 1022 keV, i.e. it “stops”, annihilation occurs with the departure of 2x gamma-quanta under 180 degrees with the same energy.
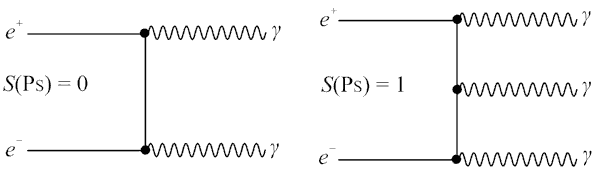
Para-positronium and ortho-positronium decay diagrams
Registration of emitted gamma-quanta allows determining the point of decay with high accuracy. An event is the simultaneous registration of 2x gamma quanta on opposite sides of a ring detector.
All isotopes used for PET are short-lived. The half-lives of the most widely used isotopes: 18F (fluorine-18) - 109 minutes, 11C (carbon-11) - 20 minutes, 13N (nitrogen-13) - 10 minutes. One of the shortest used in PET - 15O (oxygen-15) with a half-life of 122 seconds. In view of this fact, the only way to obtain isotopes for PET, with the exception of fluorine, is in situ synthesis on a cyclotron. At the word "cyclotron" I immediately recall the LHC, fortunately, medical cyclotrons for PET are much more compact. The characteristic size is 3 m, the characteristic proton energy is up to 30 MeV.

GE PETtrace 800 Cyclotron. Image from GE Healthcare official brochure
After working on the cyclotron, the isotope enters a specialized laboratory, where the required RFP is synthesized. The resulting radiopharmaceutical is subject to mandatory testing in the quality control laboratory to confirm that the substance obtained is the required radiopharmaceutical, does not contain toxins and is safe for administration to the patient. After receiving confirmation from the quality control laboratory, the radiopharmaceutical is injected into a patient and a scan is performed on a tomograph (PET / CT or PET / MRI).
One of the most common (if not the most common) RFP for PET is 18F-FDG (fluorodeoxyglucose), in fact - a glucose molecule labeled with a fluorine-18 atom. When dividing, cancer cells actively absorb glucose, respectively, if the image shows an area with a large amount of glucose, which is not typical for a healthy metabolism, then the cancer tumor is likely to grow in this area.
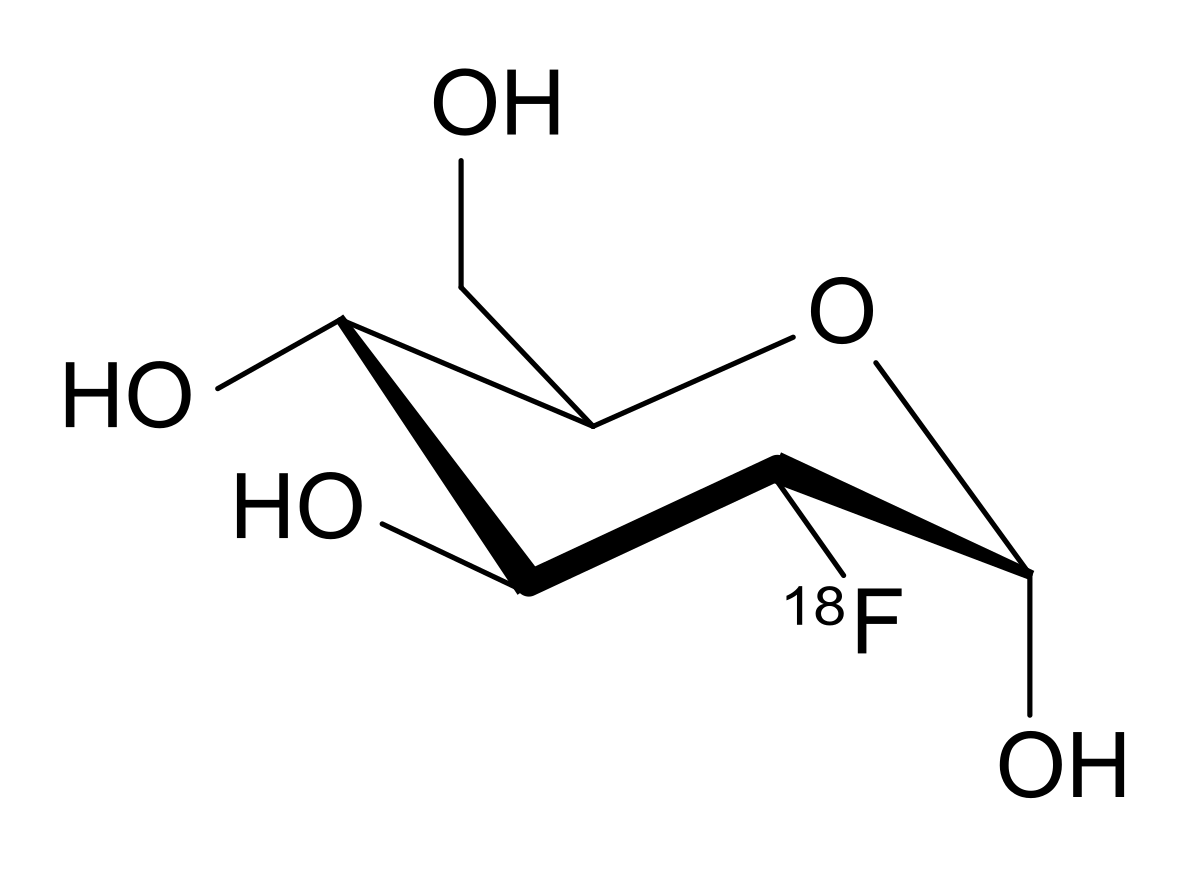
Molecule 18F-FDG. Instead of one of the OH groups, an 18F atom is attached.
It is important to note that PET is a functional method, whereas CT or MRI is anatomical. Those. if there is a tumor at very early stages, it will not yet stand out on a CT scan or MRI against the background of a healthy organ, while on PET it will already be “glowing”. Accordingly, to obtain a complete picture, it is necessary to combine two techniques - PET sees the tumor, and CT or MRI provide an accurate anatomical binding to the organ.
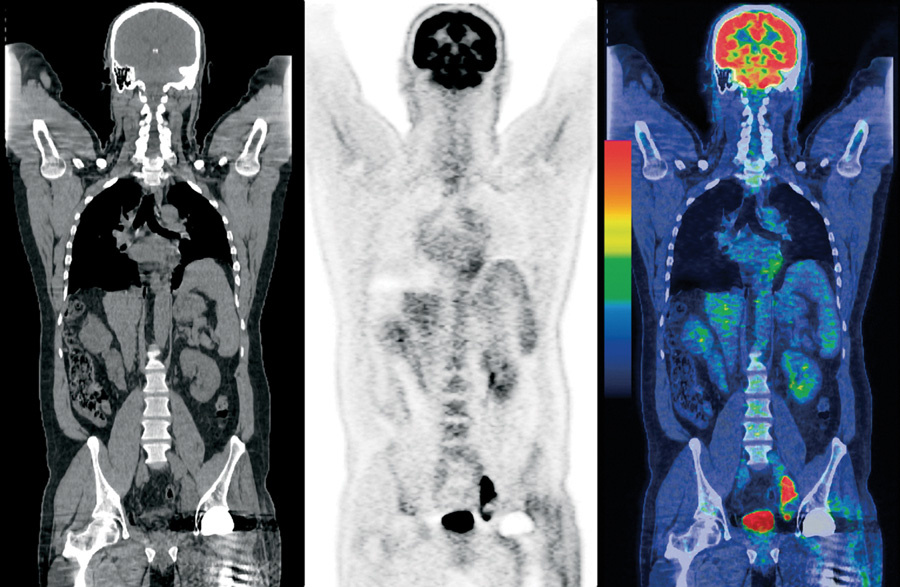
Consistently image CT, PET and PET / CT. Image from the Internet
PS: Rarely where mentioned, but the PET method is used not only in the diagnosis of cancer, but also to study the functions of internal organs. For example, the method is widely used in cardiology in the study of heart function.
The fundamental principles underlying PET
The diagnostic method is based on the fact that some substances characteristic of the human metabolism in general and cancer cells in particular are labeled with a radioactive label and then introduced into the human body. This compound is called radiopharmaceutical - RFP. The subsequent detection of decay products allows us to construct a three-dimensional map of the distribution of the label in the body to determine the absorption regions that are uncharacteristic for a healthy person. An important feature of the PET technique is that beta-plus decay is the dominant decay mechanism, i.e. decay with the formation of a positron.

A PET / CT (positron emission combined with a computer) tomograph GE Discovery 610. Image taken from the official website of GE Healthcare. Note The vertical bar at the patient's feet is the breath control system.
Here it is necessary to make a step towards quantum mechanics. Annihilation of a positron and an electron does not occur instantaneously. A positron emitted by a radioactive label, when it encounters an electron, forms a bound state - “positronium”. Both an electron and a positron are fermions, so the total spin of the bound state can be zero (para-positronium) or one (ortho-positronium). The lifetime of para-positronium is about 0.1 ns, whereas ortho-positronium is 3 orders of magnitude longer. Para-positronium can decay only into an even number of gamma-quanta, ortho-positronium, on the contrary, only into an odd number of gamma-quanta. This behavior stems from the laws of conservation of quantum mechanical parities and symmetries. In view of the low positron energies in the case of PET, it can be assumed that only 2-photon and 3-photon decays are possible. In addition, a positron in the composition of ortho-positronium, due to a much longer lifetime, can react with other electrons of the medium with a transition from the ortho-to-para state. In fact, the dominant decay mechanism is the decay with the formation of 2x gamma quanta, although from a quantum-mechanical point of view, the formation of ortho-positronium is 3 times more likely. The above is true only for dense environments, which is the human body. It is important that the emitted gamma-quanta have the same energy of 511 keV and fly away in strictly opposite directions. In the framework of quantum mechanics, this statement can be proved strictly, within the framework of the macro-world mechanics can be represented as follows: while the positronium energy exceeds 1022 keV (the total rest energy of an electron and positron), then positronium "lives and moves", losing energy when interacting with matter. As soon as the positronium energy drops to 1022 keV, i.e. it “stops”, annihilation occurs with the departure of 2x gamma-quanta under 180 degrees with the same energy.
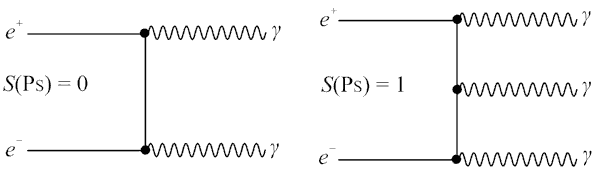
Para-positronium and ortho-positronium decay diagrams
Registration of emitted gamma-quanta allows determining the point of decay with high accuracy. An event is the simultaneous registration of 2x gamma quanta on opposite sides of a ring detector.
Isotopes
All isotopes used for PET are short-lived. The half-lives of the most widely used isotopes: 18F (fluorine-18) - 109 minutes, 11C (carbon-11) - 20 minutes, 13N (nitrogen-13) - 10 minutes. One of the shortest used in PET - 15O (oxygen-15) with a half-life of 122 seconds. In view of this fact, the only way to obtain isotopes for PET, with the exception of fluorine, is in situ synthesis on a cyclotron. At the word "cyclotron" I immediately recall the LHC, fortunately, medical cyclotrons for PET are much more compact. The characteristic size is 3 m, the characteristic proton energy is up to 30 MeV.

GE PETtrace 800 Cyclotron. Image from GE Healthcare official brochure
After working on the cyclotron, the isotope enters a specialized laboratory, where the required RFP is synthesized. The resulting radiopharmaceutical is subject to mandatory testing in the quality control laboratory to confirm that the substance obtained is the required radiopharmaceutical, does not contain toxins and is safe for administration to the patient. After receiving confirmation from the quality control laboratory, the radiopharmaceutical is injected into a patient and a scan is performed on a tomograph (PET / CT or PET / MRI).
One of the most common (if not the most common) RFP for PET is 18F-FDG (fluorodeoxyglucose), in fact - a glucose molecule labeled with a fluorine-18 atom. When dividing, cancer cells actively absorb glucose, respectively, if the image shows an area with a large amount of glucose, which is not typical for a healthy metabolism, then the cancer tumor is likely to grow in this area.

Molecule 18F-FDG. Instead of one of the OH groups, an 18F atom is attached.
Conclusion
It is important to note that PET is a functional method, whereas CT or MRI is anatomical. Those. if there is a tumor at very early stages, it will not yet stand out on a CT scan or MRI against the background of a healthy organ, while on PET it will already be “glowing”. Accordingly, to obtain a complete picture, it is necessary to combine two techniques - PET sees the tumor, and CT or MRI provide an accurate anatomical binding to the organ.

Consistently image CT, PET and PET / CT. Image from the Internet
PS: Rarely where mentioned, but the PET method is used not only in the diagnosis of cancer, but also to study the functions of internal organs. For example, the method is widely used in cardiology in the study of heart function.
All Articles