How does depression work?
Classification of depressive disorders; the role of monoamines (dopamine, norepinephrine and serotonin); mechanisms of action of antidepressants; depression, stress and neuroplasticity.

Depression (from the Latin. Deprimo - suppress) is a mental illness characterized by the “depressive triad” , including the following disorders:
1. In the emotional sphere. Depressed mood and anhedonia - the inability to derive pleasure from natural things: food, alcohol, intercourse, sex, etc.
2. In the cognitive sphere. The negative self-image, the negative experience of the world, the negative vision of the future is the so-called “cognitive triad” . The triad within the triad + person is unable to adequately assess the situation, cannot apply the previous positive experience of solving the problem.
3. In the motor sphere. As a rule, motor lethargy, but a backlash can be observed - agitated arousal: a patient in a calm atmosphere can constantly jump up, swing her arms, constantly change her posture, or, for example, stand up and leave in the middle of a conversation.

Psychiatry: national leadership. M .: GEOTAR-Media, 2009. 1000 p.
According to WHO (Fact Sheet No. 369), more than 300 million people suffer from depression, and this figure tends to increase. Depression is different from ordinary mood changes and short-term emotional reactions to problems in everyday life. It can lead to serious health problems, social maladjustment, impairment of performance and learning. In the worst cases, it can lead to suicide.
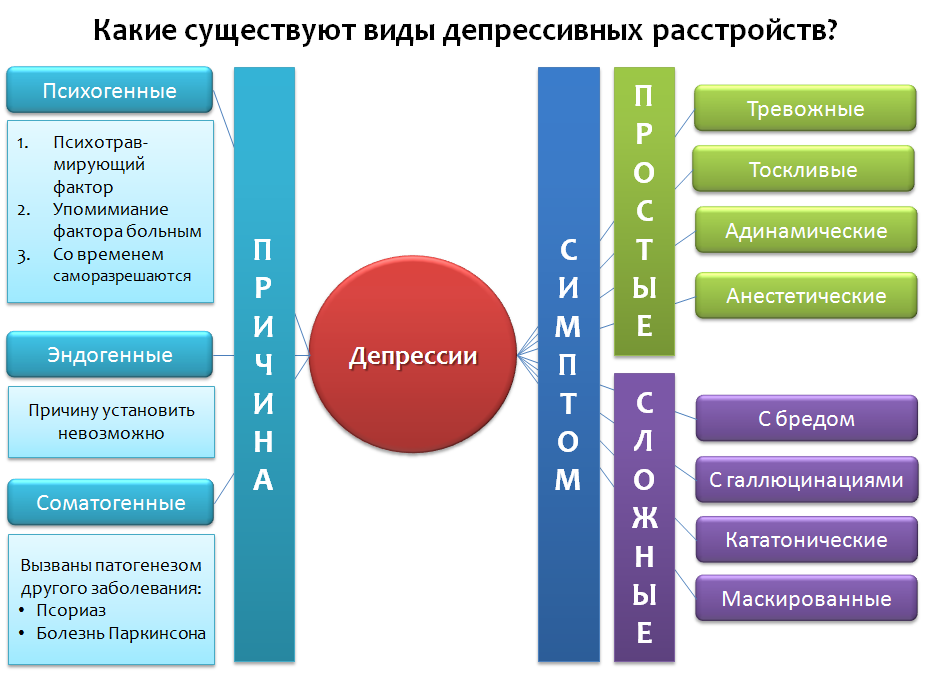
Fig. 1 Depression: The Treatment and Management of Depression in Adults (Updated Edition). Leicester (UK): British Psychological Society, 2010.
Let's take a look at the simplified classification of depressive disorders (Fig. 1), which is not absolute, but includes basic examples. Depression can be classified for the reason that caused them (etiology), and the prevalence of those or other symptoms, as well as their various combinations.
In the first case, there are three categories:
1. Psychogenic (reactive) depressions are caused by a stressful factor. This factor appears in the speech of the patient. As a rule, such depressions can be resolved independently after the passage of time, the termination of the action of the factor - “time heals”.
2. Endogenous - Depression, caused, as believed, by internal factors that can not be established.
3. Somatogenic - caused by the pathogenesis (mechanism of disease development) of various diseases. It is important not to confuse them with psychogenic. For example, if a person is depressed by the awareness of the presence of a certain disease, this is psychogenic (reactive) depression. Somatogenic include those depressions that are caused by the mechanism of another disease. For example, in Parkinson's disease, nerve cells that produce dopamine die, which, in turn, plays an important role in the emotional response. Dopamine deficiency in the areas of the brain responsible for the emotional response leads to depression. A second example is psoriasis, which can lead to a decrease in serotonin production (an important mood regulator) in the central nervous system, and thus increase the risk of developing depressive disorders.
For the predominant symptom, depression can be: anxious, dreary, adynamic, anesthetic (experiencing a lack of emotion - “emotional anesthesia”) - these are examples of “simple depression” .
"Complicated depression" combines the symptoms of depression and other psychopathologies: depression with delusions, hallucinations, catatonic, masked - the symptoms are masked as diseases of the internal organs or otherwise "somatized" - headaches, pains in the abdomen, heart, etc.
The complexity of the treatment of depressive disorders is the lack of complete recovery in case of severe or complicated forms of depressive disorders. But it is also possible to self-resolve this disease, if the course is not severe, there are no complications in the form of symptoms of other psychopathologies, etc.
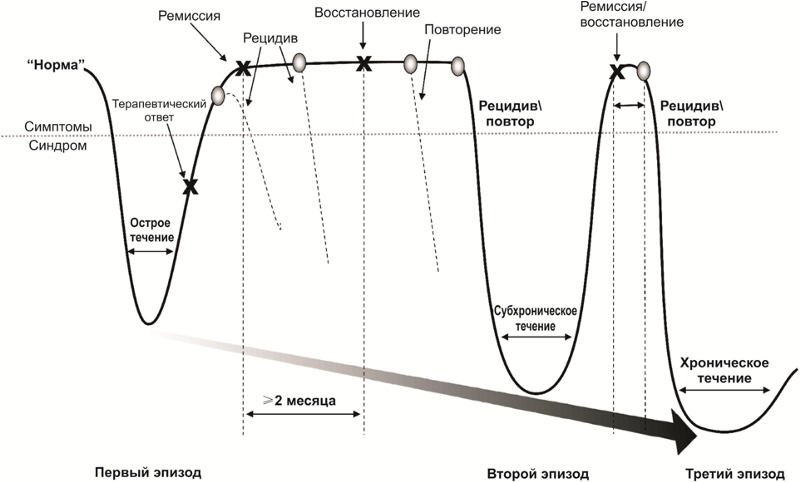
The nature of the course of depressive disorders
After a successful course of drug and psychotherapy, there comes a period of remission, which is accompanied by either a weakening (partial remission) or a complete disappearance of symptoms (complete remission) of the disease. During the period of active treatment and partial or complete remission, there is a risk of the return of symptoms of the disease - relapse.
In addition, there are risks of repeated episodes of depression after a full recovery from the first episode. Repeated episodes may be characterized by a combination of other symptoms and a more severe course. The disease may acquire a chronic course. Often, the first episode of a depressive disorder is psychogenic (reactive), and repeated (if the problem is unsolvable, the psycho-traumatic factor was too strong or protracted) - endogenous.
Figure 2 summarizes the main parameters of the monoamine theory of depression. Formulas of monoamines are given to explain the name of this group of substances - they contain only one amino group (-NH2).

Fig. 2 Monoamine Theory of Depression
* Another NH group in serotonin is not an amino group; it is part of the indole heterocycle.
It is believed that the role of monoamines in the formation of individual symptoms of depression is heterogeneous. So, for feelings of guilt and worthlessness, suicidal ideation, as well as a violation of appetite , serotonin deficiency may be responsible. Dopamine and norepinephrine are responsible for apathy, executive dysfunction and fatigue .
The lack of all monoamines in the complex indicates a depressed mood, psychomotor dysfunction and sleep disturbance.
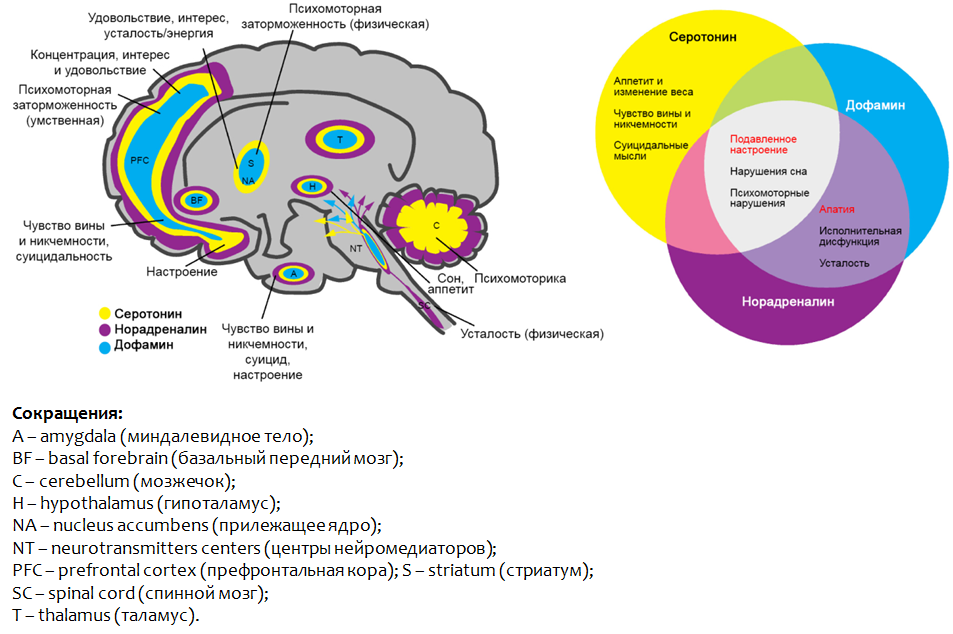
Fig.3. Saltiel PF, Silvershein DI Major depressive disorder: mechanism-based prescribing for personalized medicine // Neuropsychiatr Dis Treat. 2015. 11. P. 875–88.
Figure 3 shows the parts of the brain where the dysfunction of the monoamines presented leads to the development of depressive symptoms.
As we have said, all modern clinically effective antidepressants are created in the framework of the monoamine theory of depression.
Conventionally, the principle of action of antidepressants can be divided into two groups:
1. Means that increase the concentration of monoamines (mainly serotonin and norepinephrine) in the brain;
2. Funds that take on the function of monoamines (mainly serotonin), stimulating specific receptors.

Pic.4
Let us consider in more detail the main groups of molecular mechanisms of antidepressants. Figure 5 shows synaptic contact between two nerve cells: the top is the nerve ending of one neuron ( synapse ), the bottom is the other nerve cell that receives the signal.

Fig. 5. Drug Therapy of Depression and Anxiety Disorders. Goodman and Gilman's The Pharmacological Basis of Therapeutics. Twelfth Edition. 2011. Stahl SM Basic psychopharmacology of antidepressants. Part 1: Antidepressants have seven mechanisms // The Journal of Clinical Psychiatry. 1998. 59. Suppl 4. P. 5-14.
Neuromediators (serotonin and norepinephrine) are synthesized in nerve cells, through which cells transmit a signal to each other. The starting material for the synthesis are essential amino acids - L-tryptophan and L-phenylalanine. After synthesis, the mediators are packaged in special granules - vesicles , in which they move to the nerve endings ( synapses ) and deposited there.
After the cell has received a certain stimulus, the mediators stand out from the nerve ending (synapse) into the synaptic cleft - the gap between two nerve cells. On the surface of the cell “receiving” the signal, there are special protein formations - receptors (in this case, serotonin and adrenoreceptors) that bind to the mediator. After binding, the mediator activates (stimulates) the corresponding receptor, which leads to a change in metabolic processes inside the cell and, accordingly, changes its function (strengthens or suppresses).
After successfully completing its function, 80% of the mediator is captured back into the nerve cell, where part of the mediator is destroyed by the monoamine oxidase type A (MAO-A) enzyme, and part of it is again packaged into vesicles for reuse. Reuptake of the mediator can significantly reduce the energy costs of synthesizing a mediator from amino acids.

Fig. 6 Cons outweigh and justify the search for new hypotheses and targets.

1. Violate the reverse capture of the mediator in the nerve ending, thereby increasing its concentration in the synaptic cleft and increasing its effect on the receptors. Perhaps a separate violation of serotonin reuptake (fluoxetine, fluvoxamine, paroxetine) and norepinephrine (reboxetine, atomoxetine), and the simultaneous disruption of the capture of both mediators (amitriptyline).
2. Enhance the release of mediators from the nerve endings (mirtazapine and tianeptine, which is currently prohibited ).
3. Inhibit the activity of the enzyme MAO-A and thereby preserve the mediator from destruction (moclobemide).
4. Stimulate serotonin receptors 1 subtype (Vilazodone), activation of which is associated with relief of depressive symptoms (“good” receptors).
5. Block type 2 serotonin receptors (“bad” receptors), which are responsible for the development of anxiety and depressive symptoms (trazodone).
Currently, stress is assigned the role of one of the starting (trigger) mechanisms of affective disorders (disorders of the emotional sphere, affect), including depressive ones. It is believed that it is dangerous not a one-time and strong stressful event, but a less intense and constant impact of stress, especially everyday unpredictable stressful events. Adapting to such stressful effects is impossible, and it leads to the chronic activation of the defense and adaptation mechanisms with their subsequent exhaustion.
One of the most important components of the body's physiological stress response is the hypothalamic-pituitary-adrenal axis (Fig. 7).

Fig. 7. The Hypothalamic-Pituitary-Adrenal Hyperiphyde FP & Brown ES Disorder: A Brief Primer for Primary Care Physicians // Primary Care Companion for the Journal of Clinical Psychiatry. 2001. 3 (4). P. 151–155.
Sequential stress activation of the central structures (tonsils - hypothalamus - pituitary) leads to the production of adrenal cortex hormones - glucocorticoids (cortisol) - stress hormones. The latter are able to act on brain structures (responsible for the emotional-stress response (prefrontal cortex and hippocampus)) and disrupt neuroplasticity processes.
Violations of neuroplasticity lead to disruption of the normal connection between the structures of the brain (responsible for the emotional response).

Fig. 8 Fuchs E., Flügge G. Adult Neuroplasticity: More Than 40 Years of Research // Neural Plasticity. 2014. Article ID 541870. Doi: 10.1155 / 2014/541870; Joyce Sh. Neuroplasticity and Clinical Practice: Building Brain Power for Health // Frontiers in Psychology. 7 (2016): 1118. PMC. Web. 7 May 2017. Zilles K. Neuronal plasticity as an adaptive property of the central nervous system // Annals of Anatomy. 1992. Vol. 174. No. 5. P. 383–391.
The most important in the context of depressive disorders are the prefrontal cortex, the amygdala and the hippocampus.

Fig. 9. Gorman JM, Docherty JP A Hypothesized Role for Dendritic Remodeling in the Etiology of Mood and Anxiety Disorders // The Journal of Neuropsychiatry and Clinical Neurosciences. 2010. 22: 3. P. 256–264
Normally, when there is a complete connection between the neurons of these structures, the prefrontal cortex processes information obtained from the hippocampus (memory, emotional coloring of memories and events). The amygdala is a structure responsible for the feeling of fear. Normally, the excessive activity of this structure is suppressed by the prefrontal cortex.
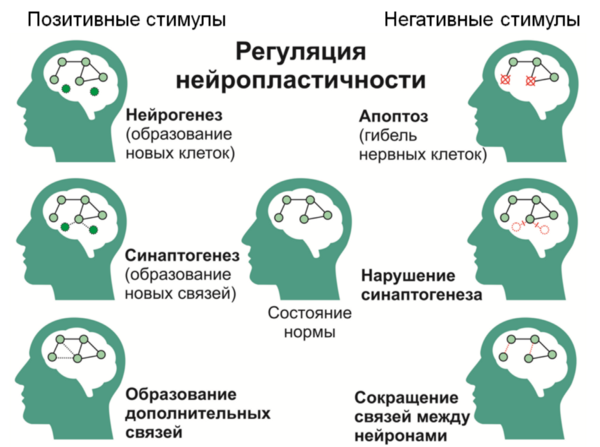
Fig. ten
It is known that against the background of depressive disorders, the processes of neuroplasticity are disturbed, in particular, the number of contacts between nerve cells decreases, the rate of impulse transmission changes, the number of neurons decreases. In addition, amid depression, a decrease in the volume of the hippocampus and the prefrontal cortex is noted. Such changes contribute to the disruption of the normal functional connection between the structures represented.
Depressive symptoms seem to be mediated by these changes: uncontrollable anxiety, which often occurs in patients with depression, may be due to the lack of inhibition of the amygdala by the prefrontal cortex.

Fig.11. Gorman JM, Docherty JP A Hypothesized Role for Dendritic Remodeling in the Etiology of Mood and Anxiety Disorders. The Journal of Neuropsychiatry and Clinical Neurosciences. 2010. 22 (3). P. 256–64. Kudryashov N.V. Experimental studying of psychotropic activity of derivatives pirazolo [c] pyridine GIZH-72 and pyrrolodiazepine GMAL-24 in the conditions of an unpredictable chronic moderate stress / dissertation of the candidate of Biological sciences. March 14, 2006 M., 2016. 198 p.
The inability to adequately assess the situation and use the previous positive experience is the result of a breakdown in the connection between the prefrontal cortex and the hippocampus. Reducing the volume of the hippocampus may explain the pathologically reduced mood.
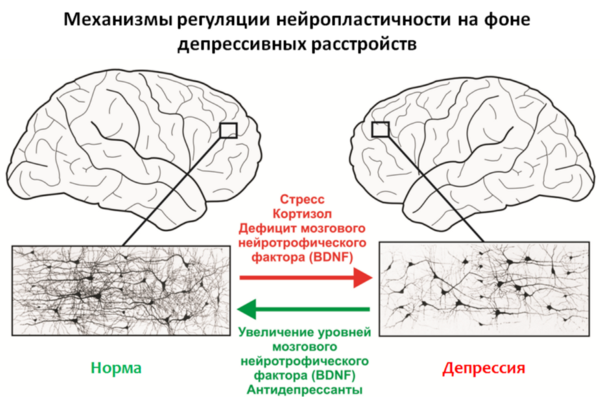
Fig.12
An important regulator of neuroplasticity processes is the brain-derived neurotrophic factor (BDNF - brain derived neurotrophic factor ), whose levels are reduced due to stress and depression.
Stress hormones such as cortisol, a glucocorticosteroid produced by the adrenal cortex, can also act as negative regulators of neuroplasticity. It is well known that the majority of antidepressants used (for chronic use) are capable of increasing levels of BDNF and, apparently, this is part of their therapeutic effect.
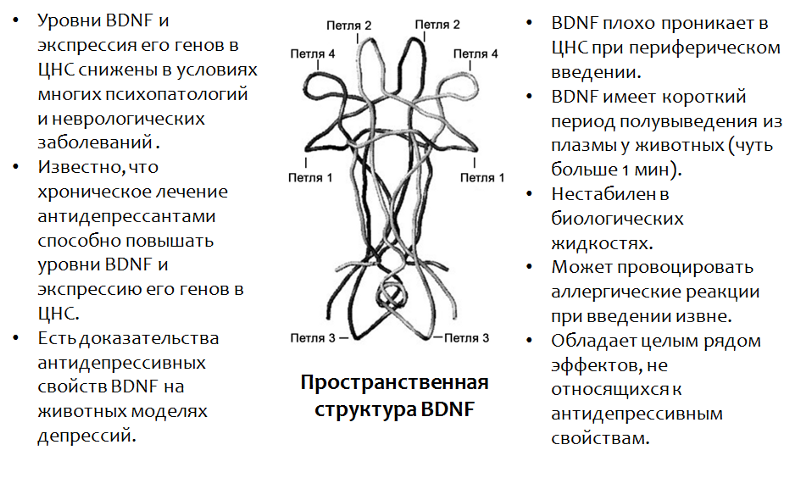
Fig.13. Castrén E., Rantamäki T. The role of BDNF and its antidepressant drug action: Reactivation of developmental plasticity // Developmental Neurobiology. 2010. 70 (5). P. 289–97.
* BDNF plays an important role in many psychopathologies, including and depressions. The use of BDNF itself is impossible due to a number of reasons (which are listed in the figure).
In addition to antidepressants, there are other factors that contribute to an increase in BDNF levels in the CNS, and they coincide with the positive stimuli of neuroplasticity - training, exercise, new experience, diet, etc. Moreover, these factors can often complement drug therapy for depressive disorders.
Figure 14 presents data on the study of the antidepressant properties of BDNF itself in animal models (rats). Since BDNF itself cannot penetrate into the brain (through the blood-brain barrier) with peripheral administration, in the BDNF experiments it was injected directly into the brain.
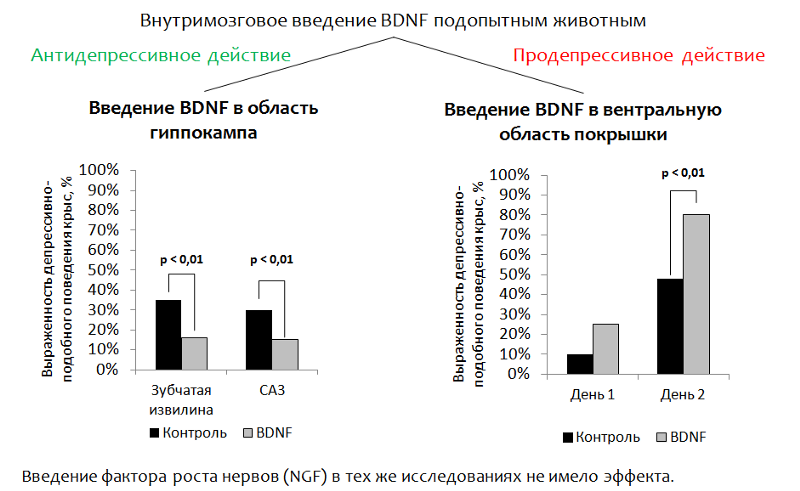
Fig.14 Eisch AJ, Bolaños CA, de Wit J. et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression // Biological Psychiatry. 2003. 54 (10). P. 994–1005; Shirayama Y., Chen AC, Nakagawa S., Russell DS, Duman RS Brain-derived neurotrophic factor produces antidepressant effects on behavioral models of depression. Journal of Neuroscience. 2002. 22 (8). P. 3251–61.
1. Introduction to the hippocampus . The main idea was to address the introduction of BDNF into the brain area responsible for neurogenesis (the dentate gyrus of the hippocampus is one of the so-called “neurogenic niches” ). After administration, depressive-like behavior of animals was evaluated. (The dysphoric component of depression is evaluated. Animals (rats or mice) are placed in a cylinder with water, from which it is impossible to get out on their own. After some time, the animal's active attempts to get out of the cylinder are replaced by a “state of despair” (the animal is in the water with almost no movement.)
Reduction of immobility (immobilization) of an animal is considered as a correlate of antidepressant effect. BDNF had an antidepressant effect after injection into the dentate gyrus (neurogenic niche) and CA3 zone of the hippocampus (the neurons of this zone ensure the interaction of the dentate gyrus with other areas of the hippocampus).
2. With the introduction of BDNF in the ventral region of the tire (the zone responsible for the production of dopamine and suffering from depressive disorders), the opposite effect was recorded - an increase in depressive-like behavior.
Since it is not possible to use BDNF itself as a medicine, a drug is developed based on this factor. In particular, the active parts of the BDNF molecule (whose spatial structure is caused by the name of the loop, Fig. 15) have been well studied.
Currently, mimetics (activity imitators) of BDNF are being actively studied.
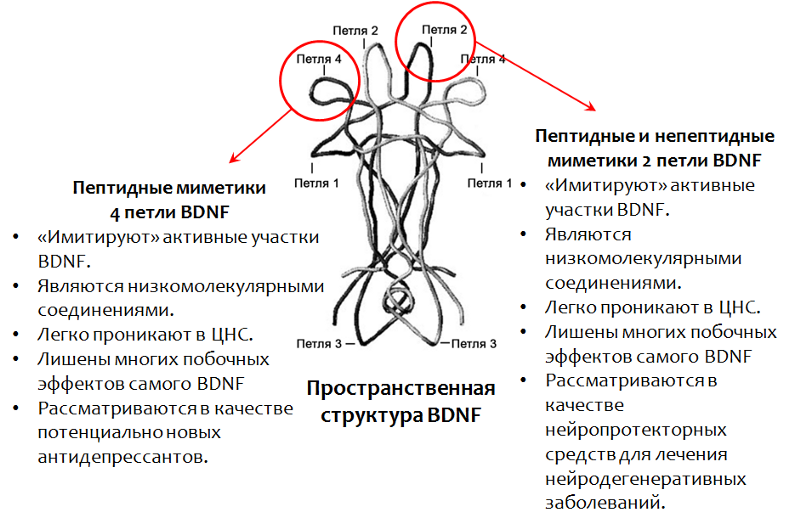
Fig.15. Fletcher JM, Morton CJ, Zwar RA et al. Design of a conformationally defined and proteolytically stable circular mimetic of brain-derived neurotrophic factor // The Journal of Biological Chemistry. 2008. 283 (48). P. 33375–83. Massa SM, Yang T., Xie Y. et al. TrkB signaling and prevent small neuronal degeneration in rodents // The Journal of Clinical Investigation. 2010. 120 (5). P. 1774–85. Seredenin S.B., Voronina T.A., Gudasheva T.A. et al. Antidepressive effect of the original low molecular weight mimetic BDNF, dimeric dipeptide GSB-106 // Acta Naturae. 2013. 4 (19). P. 116–120.

Fig. 16. Seredenin SB, Voronina T.A., Gudasheva T.A. et al. Antidepressive effect of the original low molecular weight mimetic BDNF, dimeric dipeptide GSB-106 // Acta Naturae. 2013. 4 (19). P. 116–120.
GSB-106 - a substance of peptide structure, is a mimetic of 4 BDNF loops (domestic development). The substance has an antidepressant effect on animal models with different modes of administration. Currently, extensive research is being conducted on the pharmacological properties of this compound in order to create on its basis an antidepressant of a new generation.

Fuchs E., Flügge G. Adult Neuroplasticity: More Than 40 Years of Research // Neural Plasticity. 2014. Article ID 541870, doi: 10.1155 / 2014/541870
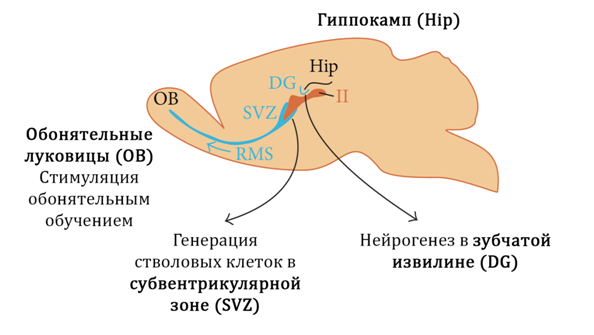
Fig. 17
In Figure 17 we show the neurogenic zones (niches) of mammals:
1) the dentate gyrus of the hippocampus
2) Olfactory bulbs
3) Subventricular zone
It is believed that in humans the main neurogenic niche is the hippocampus (dentate gyrus).
Stress, which is considered as one of the main triggering incentives for depressive disorders, leads to a decrease in BDNF levels and an increase in cortisol, which, in turn, enhances the effect of glutamate on the central nervous system.

Fig. 18
These changes, in aggregate, suppress hippocampal neurogenesis and lead to a decrease in the volume of the hippocampus. Under the action of glutamate, activation of apoptosis (programmed nerve cell death) is also possible. When neurogenesis is impaired, the brain cannot fully compensate for the losses and depressive symptoms develop.
Glutamate is one of the main excitatory amino acids of the CNS. Violation of neuroplasticity under the influence of excessive glutamate action, apparently, is associated with a compensatory reaction. Neurons "remove" unnecessary connections and die (apoptosis) in order to protect the central nervous system from over-stimulation and subsequent damaging effects of this process.
A well-known fact is the ability of antidepressants to stimulate neurogenesis, but the mechanisms underlying this phenomenon are not fully understood to date. It is known that all antidepressant groups act on the monoamine system of the brain and compensate for functional or material deficiencies of serotonin and norepinephrine. In addition, drugs of this pharmacological group increase the levels of brain neurotrophic factor.
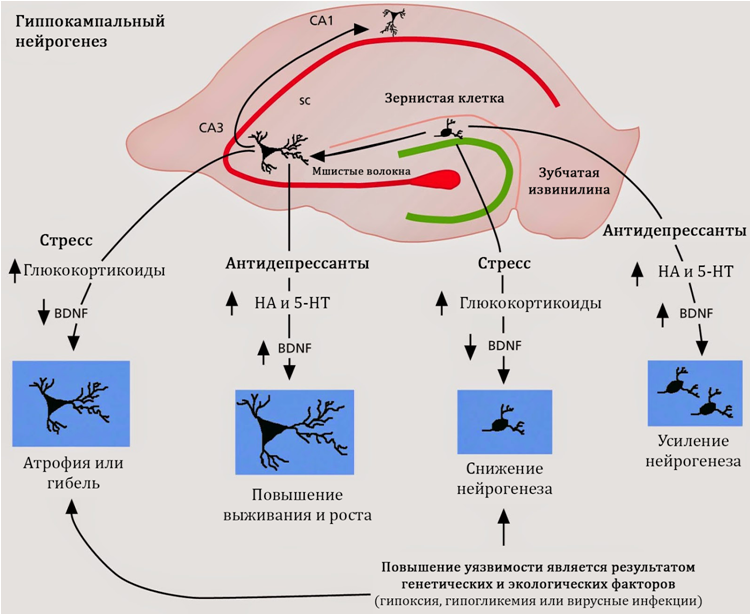
Fig. nineteen
Stimulation of neurogenesis is characteristic of antidepressants, regardless of their mechanism of action, chemical structure or class. Consequently, the search for the mechanisms of regulation of neurogenesis should be carried out in common for all antidepressants properties. Such common properties are the activity of antidepressants against serotonin and norepinephrine.
To date, an understanding of the role of serotonin in the regulation of hippocampal neurogenesis is being formed.

Fig. 20. Alenina N., Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behavioural Brain Research. 2015. 277. P. 49–57.
First, the dentate gyrus of the hippocampus receives serotonin regulation from large suture nuclei (an accumulation of nerve cells that produce serotonin; are located in the brain stem), both directly and through intercalated neurons, which carry various subtypes of serotonin receptors on their surface.
Secondly, serotonin receptors 1A of the subtype were found on the stem cells themselves , which indicates the potential of serotonin to regulate brain stem cells.

Fig. 21
As shown in experimental studies (in animals and cell cultures), selective serotonin reuptake inhibitors (SSRIs, the classic drug from this group, fluoxetine) are able to stimulate the proliferation stage of neurogenesis in the hippocampus.
The proposed mechanism is an increase in the concentration of serotonin (5-NT - serotonin, also known as 5-hydroxytryptamine) in the central nervous system and the subsequent (enhanced) stimulation of serotonin by neurogenesis. Serotonin receptors of the 1A subtype (5HT1A receptors) may also serve as
potential targets of antidepressants during neurogenesis. These assumptions are consistent with the data on the positive (therapeutic) effect of serotonin 5-HT1A receptor activation by antidepressants (for example, Vilazodone) against depressive disorders.
Another argument that allows us to consider the stimulation of neurogenesis as the main mechanism of action of antidepressants is the coincidence in time between the average time of onset of the therapeutic effect (from 2 to 7 weeks) and the full cycle of neurogenesis (3-7 weeks).
In addition to these mechanisms, antidepressants from the SSRI group also demonstrate the ability to increase levels of BDNF, but the mechanisms of this effect remain unknown.

Fig. 22. Perera TD, Dwork AJ, Keegan KA, et al. Nonhuman Primates // PLoS ONE. Necessity of Hippocampal Neurogenesis for the Adult Nonhuman Primates // PLoS ONE. 2011. 6 (4): e17600. doi: 10.1371 / journal.pone.0017600.
Studies on primates (the most relevant animal model) showed the ability of fluoxetine (torg. Prozac) to stimulate neurogenesis under stress (in this case, the model of insulating stress was used). Figure 22 shows that fluoxetine significantly increased (statistically significantly) the rate of proliferation (division) of stem nerve cells in the hippocampus of primates.

Fig. 23. Perera TD, Dwork AJ, Keegan KA, et al. Nonhuman Primates // PLoS ONE. Necessity of Hippocampal Neurogenesis for the Adult Nonhuman Primates // PLoS ONE. 2011. 6 (4): e17600. doi: 10.1371 / journal.pone.0017600.
The impact of stress led to a decrease in the granular layer of the dentate gyrus of the hippocampus - the main neurogenic zone of the primate's brain. The introduction of fluoxetine against a stressful exposure prevented this change and maintained normal volume of this structure (total volume).
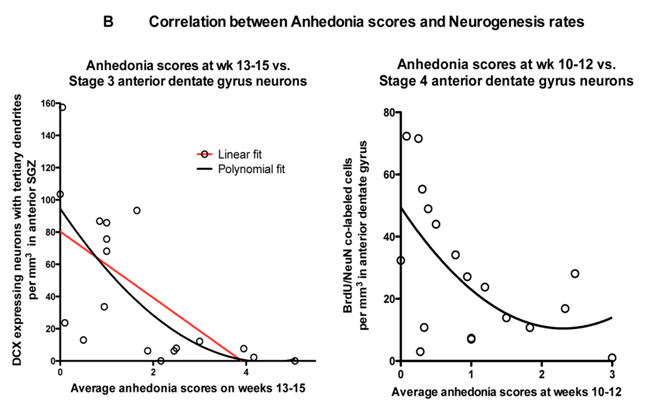
Perera TD, Dwork AJ, Keegan KA, et al. Necessity of Hippocampal Neurogenesis for the Non-Primary. PLoS ONE. 2011. 6 (4): e17600. doi: 10.1371 / journal.pone.0017600.
A correlation was found between the stimulation of neurogenesis with fluoxetine and the prevention of the development of depressive behavior (anhedonia).
When studying the effect of fluoxitin on the neurogenesis of nonhuman primates, a significant correlation was established between a decrease in neurogenesis (caused by stress) and an increase in depressive-like behavior in primates (anhedonia, which was determined by the combination of social and eating disorders). -like behavior.
Norepinephrine is another important monoamine that, along with serotonin, is able to participate in the regulation of hippocampal neurogenesis.

Fig.25. Jhaveri DJ, Mackay EW, Hamlin AS, et al. Norepinephrine directly activates adult hippocampal precursors via the β3 adrenergic receptors // The Journal of Neuroscience. 2010. 30 (7). H. 2795–2806. doi: 10.1523 / JNEUROSCI.3780–09.2010.
Studies on hippocampal neuron cultures have shown that norepinephrine (unlike serotonin) increased the number of stem cells. Serotonin, as demonstrated earlier, did not affect the quantity, but the rate of proliferation.
In addition to quantitative changes, norepinephrine caused and qualitative - increased the size of the neurospheres, which is clearly shown in the electron microscope image (see fig.26).

Fig. 26. Jhaveri DJ, Mackay EW, Hamlin AS, et al. Norepinephrine directly activates adult hippocampal precursors via the β3 adrenergic receptors // The Journal of Neuroscience. 2010. 30 (7). H. 2795–2806. doi: 10.1523 / JNEUROSCI.3780–09.2010.
Earlier, we considered changes in neurons, but ignored the role of glial cells in the formation of depressive disorders. However, experimental and clinical studies indicate the possible role of the pathology of glial cells in the pathogenesis of depression.

Fig. 27. Rajkowska G., Miguel-Hidalgo JJ Gliogenesis and Glial Pathology in Depression // CNS & Neurological Disorders Drug Targets. 2007. 6 (3). P. 219–233.
The researchers presented a scheme for the pathogenesis of depressive disorders with the participation of glia (Fig. 27).
Genetic predisposition may include: a hereditary factor (the presence of a depressive disorder in one of the parents increases the risk of this disease in a child); gene polymorphism: BDNF, a serotonin carrier (which is involved in the reuptake of serotonin into nerve and glial cells), serotonin receptors, serotonin synthesis enzymes (tryptophan hydroxylase type 2).
Genetic vulnerability in combination with environmental and stress factors creates a favorable environment for the formation of depressive disorder.
Scientists have established that the role of glial cells is not the same in young and elderly patients (Fig. 28). Glial cells may play an important role in the pathogenesis of the early stages of depressive disorders, which can cause a marked decrease in the number of pyramidal neurons in more mature age.

Fig. 28. Rajkowska G., Miguel-Hidalgo JJ Gliogenesis and Glial Pathology in Depression // CNS & Neurological Disorders Drug Targets. 2007. 6 (3). P. 219–233.
In particular, the loss of function of glial cells at a young age increases the risk of repeated episodes of depression in the elderly, but the deficit of pyramidal neurons rather than glial cells will prevail.

Fig. 29. Rajkowska G., Miguel-Hidalgo JJ Gliogenesis and Glial Pathology in Depression // CNS & Neurological Disorders Drug Targets. 2007. 6 (3). P. 219–233.
Such a dynamics is consistent with one of the most important functions of glial cells - the absorption of an excess of glutamate from the synaptic cleft (contact between nerve cells). Glutamate is one of the main excitatory neurotransmitters of the CNS and its excessive action can lead to disruption of neuroplasticity and excitotoxicity (neurotoxicity associated with excessive excitation; apparently, a protective reaction of nerve cells from overexcitation - the number of neurons and connections between them decreases).
In glial cells there is a protein transporter, which is involved in the transfer of glutamate from the synaptic cleft into the glial cell, where glutamate is metabolized.
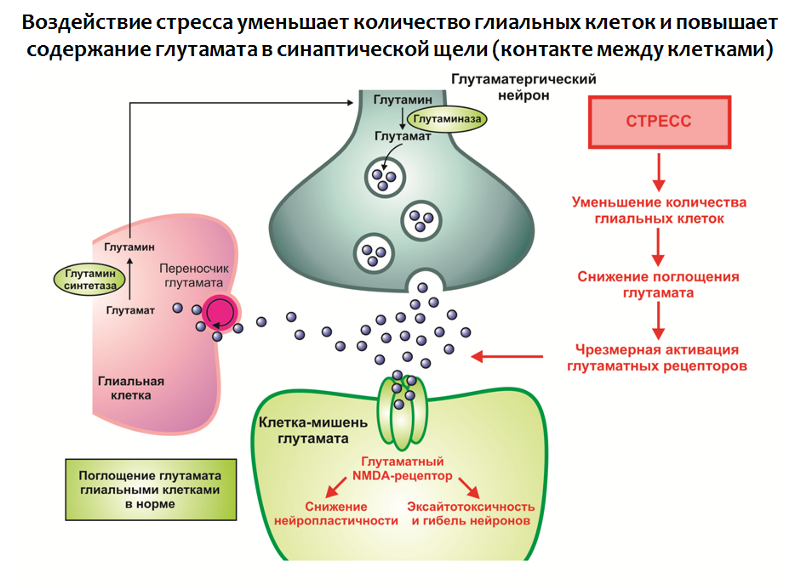
Fig. 30. Rajkowska G., Miguel-Hidalgo JJ Gliogenesis and Glial Pathology in Depression // CNS & Neurological Disorders Drug Targets. 2007. 6 (3). P. 219–233.
The impact of stress in combination with other factors leads to:
1. A decrease in the number of glial cells in the structures of the limbic system of the brain;
2. Hyperproduction of glutamate.
In this way, an excess of this excitatory neurotransmitter is formed, which is a negative modulator of neuroplasticity (as it may be part of a compensatory reaction that protects the nervous system from over-stimulation).
The function of glial cells is not limited to the absorption of glutamate, they are also involved in the production of neurotrophins, in particular BDNF (Fig. 31).
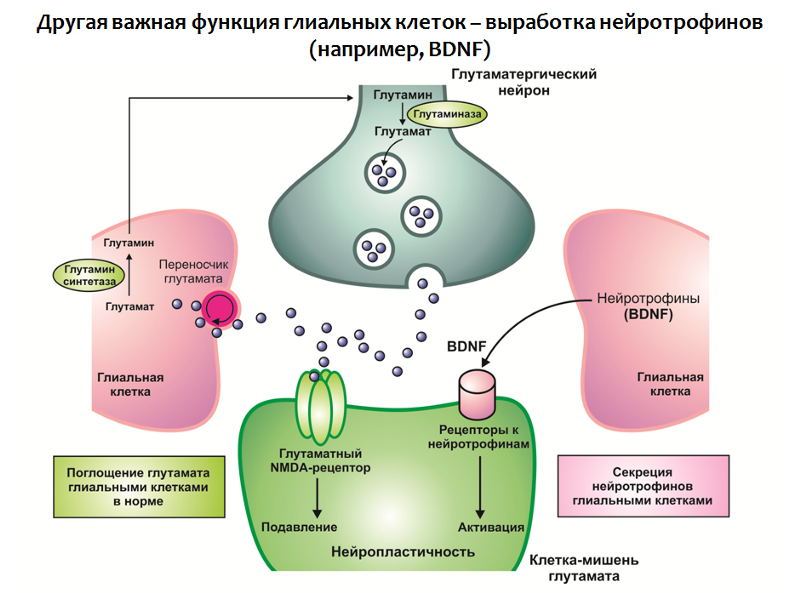
Fig. 31
Taken together, a deficit of neurotrophins and an excess of glutamate lead to impaired neuroplasticity and depressive-like changes (decrease in the volume of the hippocampus and the prefrontal cortex, disruption of the normal functional connection between the structures of the limbic circle).
In the context of this concept, it is also possible to find explanations for the therapeutic efficacy of antidepressants (Fig. 32):
1. Antidepressants can “soften” the effect of stress by normalizing the activity of the hypothalamic-pituitary-adrenal axis;
2. Increase the concentration of BDNF in the central nervous system;
3. Stimulate the processes of neuroplasticity.

Fig. 32
Figure 33 presents a generalized depressive disorder scheme that is based on the concept of stress-mediated neurodegeneration. It is seen that antidepressants occupy a niche "proofreaders of the effects of stress." With all the advantages and therapeutic potential available, antidepressants are not always effective in eliminating depressive symptoms.
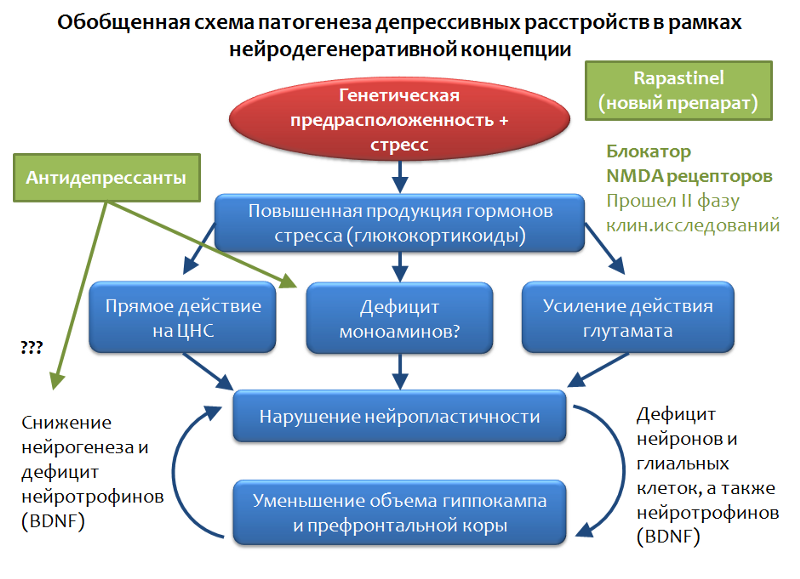
Fig. 33
There are so-called pharmacotherapy-resistant forms of depressive disorders. This phenomenon is attempted to be explained both by the diversity of stress factors, and by the different strength and duration of stress exposure, and by individual characteristics (mutation of targets of antidepressants). But the general conclusion from this situation is one - the search for fundamentally new targets for the pharmacotherapy of depressive disorders is needed.
A promising direction is the impact on the system of glutamate, if we consider this neurotransmitter as one of the key elements of the pathogenesis of depressive disorders. Within this area, significant success has been achieved - a fundamentally new antidepressant has been created, which, by its mechanism, is a glutamate blocker for NMDA receptors and prevents the excessive activity of this amino acid. Rapasintel antidepressant has now successfully completed phase I and phase II clinical trials, where it has been shown to be highly effective and is considered as a treatment for resistant forms of depressive disorders.
In the framework of the glutamatergic theory of depressive disorders, the role of the main inhibitory mediator of the central nervous system - gamma-aminobutyric acid (GABA or GABA) can be considered.
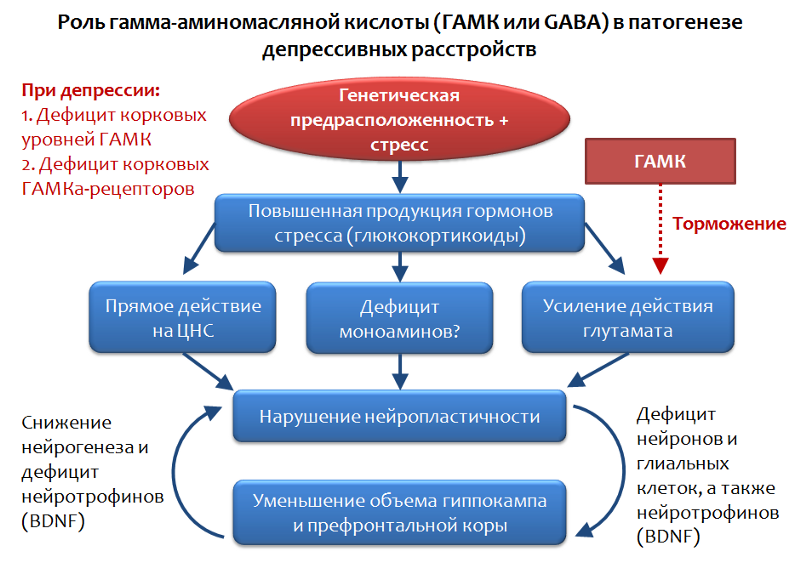
Fig. 34. Möhler H. The GABA system in anxiety and its therapeutic potential // Neuropharmacology. 2012. Jan. 62 (1). P. 42–53.
GABA is the functional opposite of glutamate and is capable of limiting its stimulating effect; therefore, an assessment of the role of GABA in depressive disorders appears to be quite logical.
It has been established that, against the background of depressive disorders, there is a shortage of cortical levels of GABA and its receptors. In particular, pyramidal neurons that produce glutamate can be inhibited by the inhibitory effects of interneurons that produce GABA. GABA realizes its inhibitory action through the activation of the GABA-A receptor.
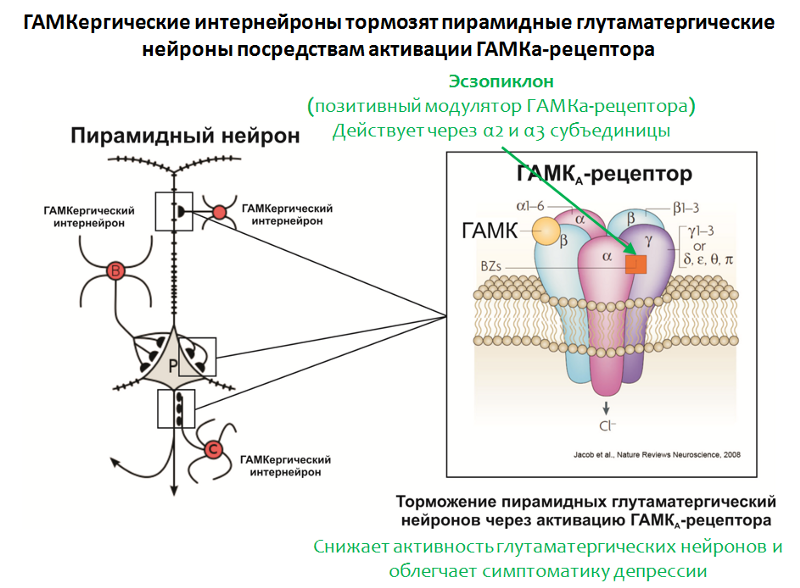
Fig. 35. Möhler H. The GABA system in anxiety and its therapeutic potential // Neuropharmacology. 2012. Jan. 62 (1). P. 42–53.
The structure of the GABA-A receptor is shown in Figure 35. The receptor consists of 5 subunits (2α, 2β and γ), each subunit has a subtype, for example, 6 variants of α-subunits are known. The combination of different subunit variants determines the GABA-A receptor subtype.
In confirmation of the role of GABA, the effectiveness of the positive modulator of the GABA-A receptor, eszopiclone, also speaks. The target of this drug is GABA-A receptors, carrying in its composition the α2- and α3-subunits. Eszopiclonesometimes used in combination with antidepressants and significantly relieves depressive symptoms even after the withdrawal of antidepressants. It is believed that its therapeutic effect is associated with a weakening of the function of glutamate. Interestingly, other positive modulators of the GABA-A receptor (for which other variants of α subunits, such as zolpidem , are necessary ) do not possess this activity.
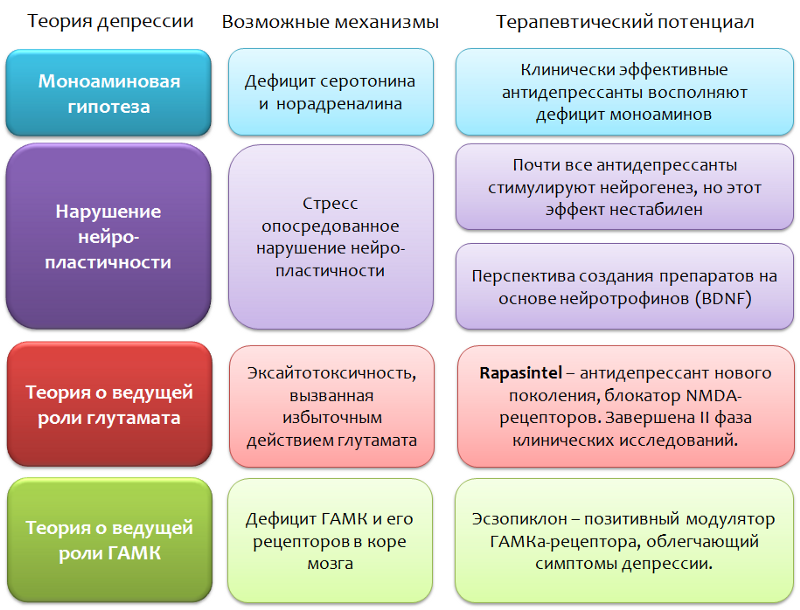
Fig. 36
And so, in the presented scheme (Fig. 37) we summarize the data on the impairment of the processes of neuroplasticity and the development of depressive symptoms.
1) The suppression of neuroplasticity processes is not strictly specific for depressive disorders, but is also observed in other psychopathologies (schizophrenia, bipolar affective disorder) and neurological diseases (multiple sclerosis, Parkinson's disease, Alzheimer's disease).
2) In animal models, the suppression of stem cell proliferation by chemical agents does not block the effects of antidepressants (unlike x-rays, where the opposite effect is noted).
3) Resource neurogenesis may be limited, and excessive stimulation can lead to depletion.
4) The long-term effects of prolonged artificial stimulation of neurogenesis are unknown. Is there a risk of developing a tumor?
5) Disruption of neuroplasticity is not an exhaustive concept of depressive disorders. The concept cannot fully explain the presence of ALL symptoms of the disease (for example, somatization of depressive symptoms, when symptoms of depression are masked as diseases of internal organs - headache, pain in the heart, abdomen, etc.), nature of the course (cyclical) and resistance some forms of depression to drug therapy (despite the fact that antidepressants activate neurogenesis and increase BDNF).
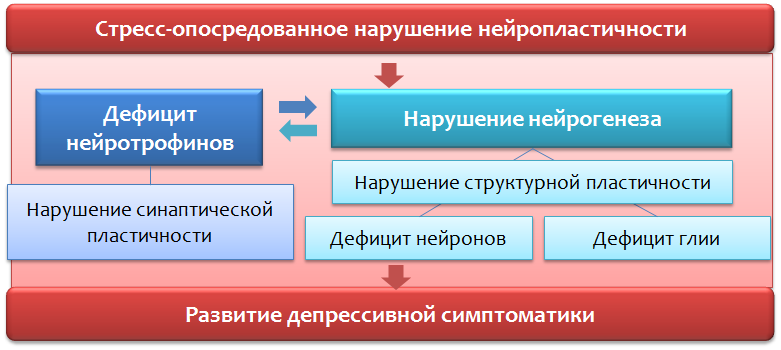
Fig. 37
- Suppression of neuroplasticity processes is not specific for depressive disorders.
- In animal models, suppression of stem cell proliferation does not always block the effects of antidepressants.
- Resource neurogenesis may be limited, and excessive stimulation can lead to depletion.
- The long-term effects of long-term "violent" stimulation of neurogenesis are unknown. Is there a risk of developing a tumor?
- Violation of neuroplasticity is not an exhaustive concept of depressive disorders.
Theories about the role of neurotransmitter amino acids - glutamate and GABA are not exhaustive. First, the presented systems (glutamatergic and GABAergic) cannot be considered in isolation from other factors, since in fact, they are an intermediate in the pathogenesis of depressive disorders or individual symptoms. Secondly, changes in the glutamatergic and GABAergic systems of the brain are observed not only in depressive, but also in a number of other disorders and conditions (schizophrenia, anxiety disorders, panic attacks, epilepsy, pain sensitivity, Parkinson's disease, Alzheimer's disease).

Fig. 38
If we consider pharmacological intervention in the glutamatergic system, then it is undoubtedly promising and even innovative, since previously, all antidepressants acted solely on the monoaminegic system of the brain. However, such a wide functional of glutamate in the central nervous system can lead to the development of undesirable effects and a number of contraindications. But it is too early to make predictions; the blocker of glutamate receptors has yet to go through phase III of clinical trials. The drug is not considered as a replacement for modern antidepressants, but as a means of complementary therapy (for example, in case of stable forms of depression).
Drugs, which are positive modulators of GABA-receptors, are not currently regarded as independent antidepressants, they are useful in eliminating certain symptoms of depressive disorders.
A promising direction for the development of new effective and safe antidepressants is the study of the mechanisms of stress itself, since it is the stress factor (stressful events) that is considered as the main starting stimulus for the formation of depressive disorders.
The search and creation of the “ideal” anti-depressant should be based on two simple principles (according to Franco Borsini):
1. The drug should not change the psyche of a healthy person.
2. The drug should act only in terms of psychopathology.

Fig. 39. Future directions // World Journal of Pharmacology. 2012. 1 (1). P. 21–29.
Interrupting the mechanisms of stress in the early stages would prevent all those changes that were considered in the context of our article. This method of correction, in theory, seems to be the most effective not only to prevent the development of depression, but also to provide reliable protection against relapses and repeated, more severe episodes.
Drug therapy, by itself, is not the only means of correcting the psychopathology in question. No less importance should be given to communication with patients, identifying the cause of the disease. In some cases, the effectiveness of psychotherapy also has a high potential, because helps to find solutions to the problem, rather than struggling with the symptoms of the disease, leaving the problem unresolved and translating depression into subchronic and chronic forms.

Depression (from the Latin. Deprimo - suppress) is a mental illness characterized by the “depressive triad” , including the following disorders:
1. In the emotional sphere. Depressed mood and anhedonia - the inability to derive pleasure from natural things: food, alcohol, intercourse, sex, etc.
2. In the cognitive sphere. The negative self-image, the negative experience of the world, the negative vision of the future is the so-called “cognitive triad” . The triad within the triad + person is unable to adequately assess the situation, cannot apply the previous positive experience of solving the problem.
3. In the motor sphere. As a rule, motor lethargy, but a backlash can be observed - agitated arousal: a patient in a calm atmosphere can constantly jump up, swing her arms, constantly change her posture, or, for example, stand up and leave in the middle of a conversation.

Psychiatry: national leadership. M .: GEOTAR-Media, 2009. 1000 p.
There are additional symptoms: loss or increased appetite (weight loss or weight gain), sleep disturbances (drowsiness or insomnia), fatigue, fatigue, etc.
According to WHO (Fact Sheet No. 369), more than 300 million people suffer from depression, and this figure tends to increase. Depression is different from ordinary mood changes and short-term emotional reactions to problems in everyday life. It can lead to serious health problems, social maladjustment, impairment of performance and learning. In the worst cases, it can lead to suicide.
Each year, about 800,000 people die as a result of suicide - the second leading cause of death among people aged 15-29 years.

Fig. 1 Depression: The Treatment and Management of Depression in Adults (Updated Edition). Leicester (UK): British Psychological Society, 2010.
Let's take a look at the simplified classification of depressive disorders (Fig. 1), which is not absolute, but includes basic examples. Depression can be classified for the reason that caused them (etiology), and the prevalence of those or other symptoms, as well as their various combinations.
In the first case, there are three categories:
1. Psychogenic (reactive) depressions are caused by a stressful factor. This factor appears in the speech of the patient. As a rule, such depressions can be resolved independently after the passage of time, the termination of the action of the factor - “time heals”.
2. Endogenous - Depression, caused, as believed, by internal factors that can not be established.
3. Somatogenic - caused by the pathogenesis (mechanism of disease development) of various diseases. It is important not to confuse them with psychogenic. For example, if a person is depressed by the awareness of the presence of a certain disease, this is psychogenic (reactive) depression. Somatogenic include those depressions that are caused by the mechanism of another disease. For example, in Parkinson's disease, nerve cells that produce dopamine die, which, in turn, plays an important role in the emotional response. Dopamine deficiency in the areas of the brain responsible for the emotional response leads to depression. A second example is psoriasis, which can lead to a decrease in serotonin production (an important mood regulator) in the central nervous system, and thus increase the risk of developing depressive disorders.
For the predominant symptom, depression can be: anxious, dreary, adynamic, anesthetic (experiencing a lack of emotion - “emotional anesthesia”) - these are examples of “simple depression” .
"Complicated depression" combines the symptoms of depression and other psychopathologies: depression with delusions, hallucinations, catatonic, masked - the symptoms are masked as diseases of the internal organs or otherwise "somatized" - headaches, pains in the abdomen, heart, etc.
The complexity of the treatment of depressive disorders is the lack of complete recovery in case of severe or complicated forms of depressive disorders. But it is also possible to self-resolve this disease, if the course is not severe, there are no complications in the form of symptoms of other psychopathologies, etc.

The nature of the course of depressive disorders
After a successful course of drug and psychotherapy, there comes a period of remission, which is accompanied by either a weakening (partial remission) or a complete disappearance of symptoms (complete remission) of the disease. During the period of active treatment and partial or complete remission, there is a risk of the return of symptoms of the disease - relapse.
In addition, there are risks of repeated episodes of depression after a full recovery from the first episode. Repeated episodes may be characterized by a combination of other symptoms and a more severe course. The disease may acquire a chronic course. Often, the first episode of a depressive disorder is psychogenic (reactive), and repeated (if the problem is unsolvable, the psycho-traumatic factor was too strong or protracted) - endogenous.
Serotonin, norepinephrine and dopamine
Figure 2 summarizes the main parameters of the monoamine theory of depression. Formulas of monoamines are given to explain the name of this group of substances - they contain only one amino group (-NH2).
The role of monoamines in the development of depressive symptoms

Fig. 2 Monoamine Theory of Depression
* Another NH group in serotonin is not an amino group; it is part of the indole heterocycle.
It is believed that the role of monoamines in the formation of individual symptoms of depression is heterogeneous. So, for feelings of guilt and worthlessness, suicidal ideation, as well as a violation of appetite , serotonin deficiency may be responsible. Dopamine and norepinephrine are responsible for apathy, executive dysfunction and fatigue .
The lack of all monoamines in the complex indicates a depressed mood, psychomotor dysfunction and sleep disturbance.

Fig.3. Saltiel PF, Silvershein DI Major depressive disorder: mechanism-based prescribing for personalized medicine // Neuropsychiatr Dis Treat. 2015. 11. P. 875–88.
Figure 3 shows the parts of the brain where the dysfunction of the monoamines presented leads to the development of depressive symptoms.
As we have said, all modern clinically effective antidepressants are created in the framework of the monoamine theory of depression.
Antidepressants
Conventionally, the principle of action of antidepressants can be divided into two groups:
1. Means that increase the concentration of monoamines (mainly serotonin and norepinephrine) in the brain;
2. Funds that take on the function of monoamines (mainly serotonin), stimulating specific receptors.

Pic.4
Let us consider in more detail the main groups of molecular mechanisms of antidepressants. Figure 5 shows synaptic contact between two nerve cells: the top is the nerve ending of one neuron ( synapse ), the bottom is the other nerve cell that receives the signal.
The main molecular mechanisms of antidepressants in the context of the monoamine hypothesis of depression

Fig. 5. Drug Therapy of Depression and Anxiety Disorders. Goodman and Gilman's The Pharmacological Basis of Therapeutics. Twelfth Edition. 2011. Stahl SM Basic psychopharmacology of antidepressants. Part 1: Antidepressants have seven mechanisms // The Journal of Clinical Psychiatry. 1998. 59. Suppl 4. P. 5-14.
Neuromediators (serotonin and norepinephrine) are synthesized in nerve cells, through which cells transmit a signal to each other. The starting material for the synthesis are essential amino acids - L-tryptophan and L-phenylalanine. After synthesis, the mediators are packaged in special granules - vesicles , in which they move to the nerve endings ( synapses ) and deposited there.
After the cell has received a certain stimulus, the mediators stand out from the nerve ending (synapse) into the synaptic cleft - the gap between two nerve cells. On the surface of the cell “receiving” the signal, there are special protein formations - receptors (in this case, serotonin and adrenoreceptors) that bind to the mediator. After binding, the mediator activates (stimulates) the corresponding receptor, which leads to a change in metabolic processes inside the cell and, accordingly, changes its function (strengthens or suppresses).
After successfully completing its function, 80% of the mediator is captured back into the nerve cell, where part of the mediator is destroyed by the monoamine oxidase type A (MAO-A) enzyme, and part of it is again packaged into vesicles for reuse. Reuptake of the mediator can significantly reduce the energy costs of synthesizing a mediator from amino acids.

Fig. 6 Cons outweigh and justify the search for new hypotheses and targets.
Briefly about how antidepressants act

1. Violate the reverse capture of the mediator in the nerve ending, thereby increasing its concentration in the synaptic cleft and increasing its effect on the receptors. Perhaps a separate violation of serotonin reuptake (fluoxetine, fluvoxamine, paroxetine) and norepinephrine (reboxetine, atomoxetine), and the simultaneous disruption of the capture of both mediators (amitriptyline).
2. Enhance the release of mediators from the nerve endings (mirtazapine and tianeptine, which is currently prohibited ).
3. Inhibit the activity of the enzyme MAO-A and thereby preserve the mediator from destruction (moclobemide).
4. Stimulate serotonin receptors 1 subtype (Vilazodone), activation of which is associated with relief of depressive symptoms (“good” receptors).
5. Block type 2 serotonin receptors (“bad” receptors), which are responsible for the development of anxiety and depressive symptoms (trazodone).
Depression and stress
Currently, stress is assigned the role of one of the starting (trigger) mechanisms of affective disorders (disorders of the emotional sphere, affect), including depressive ones. It is believed that it is dangerous not a one-time and strong stressful event, but a less intense and constant impact of stress, especially everyday unpredictable stressful events. Adapting to such stressful effects is impossible, and it leads to the chronic activation of the defense and adaptation mechanisms with their subsequent exhaustion.
One of the most important components of the body's physiological stress response is the hypothalamic-pituitary-adrenal axis (Fig. 7).

Fig. 7. The Hypothalamic-Pituitary-Adrenal Hyperiphyde FP & Brown ES Disorder: A Brief Primer for Primary Care Physicians // Primary Care Companion for the Journal of Clinical Psychiatry. 2001. 3 (4). P. 151–155.
Sequential stress activation of the central structures (tonsils - hypothalamus - pituitary) leads to the production of adrenal cortex hormones - glucocorticoids (cortisol) - stress hormones. The latter are able to act on brain structures (responsible for the emotional-stress response (prefrontal cortex and hippocampus)) and disrupt neuroplasticity processes.
Neuroplasticity disorders *
Violations of neuroplasticity lead to disruption of the normal connection between the structures of the brain (responsible for the emotional response).
* Neuroplasticity is the ability of the brain to adapt to changes through reorganization, during normal development and in conditions of pathology.

Fig. 8 Fuchs E., Flügge G. Adult Neuroplasticity: More Than 40 Years of Research // Neural Plasticity. 2014. Article ID 541870. Doi: 10.1155 / 2014/541870; Joyce Sh. Neuroplasticity and Clinical Practice: Building Brain Power for Health // Frontiers in Psychology. 7 (2016): 1118. PMC. Web. 7 May 2017. Zilles K. Neuronal plasticity as an adaptive property of the central nervous system // Annals of Anatomy. 1992. Vol. 174. No. 5. P. 383–391.
The most important in the context of depressive disorders are the prefrontal cortex, the amygdala and the hippocampus.
The interaction of brain structures is normal

Fig. 9. Gorman JM, Docherty JP A Hypothesized Role for Dendritic Remodeling in the Etiology of Mood and Anxiety Disorders // The Journal of Neuropsychiatry and Clinical Neurosciences. 2010. 22: 3. P. 256–264
Normally, when there is a complete connection between the neurons of these structures, the prefrontal cortex processes information obtained from the hippocampus (memory, emotional coloring of memories and events). The amygdala is a structure responsible for the feeling of fear. Normally, the excessive activity of this structure is suppressed by the prefrontal cortex.

Fig. ten
It is known that against the background of depressive disorders, the processes of neuroplasticity are disturbed, in particular, the number of contacts between nerve cells decreases, the rate of impulse transmission changes, the number of neurons decreases. In addition, amid depression, a decrease in the volume of the hippocampus and the prefrontal cortex is noted. Such changes contribute to the disruption of the normal functional connection between the structures represented.
Depressive symptoms seem to be mediated by these changes: uncontrollable anxiety, which often occurs in patients with depression, may be due to the lack of inhibition of the amygdala by the prefrontal cortex.
Interaction of brain structures in depression (theory)

Fig.11. Gorman JM, Docherty JP A Hypothesized Role for Dendritic Remodeling in the Etiology of Mood and Anxiety Disorders. The Journal of Neuropsychiatry and Clinical Neurosciences. 2010. 22 (3). P. 256–64. Kudryashov N.V. Experimental studying of psychotropic activity of derivatives pirazolo [c] pyridine GIZH-72 and pyrrolodiazepine GMAL-24 in the conditions of an unpredictable chronic moderate stress / dissertation of the candidate of Biological sciences. March 14, 2006 M., 2016. 198 p.
The inability to adequately assess the situation and use the previous positive experience is the result of a breakdown in the connection between the prefrontal cortex and the hippocampus. Reducing the volume of the hippocampus may explain the pathologically reduced mood.
Mechanisms of regulation of neuroplasticity on the background of depressive disorders

Fig.12
An important regulator of neuroplasticity processes is the brain-derived neurotrophic factor (BDNF - brain derived neurotrophic factor ), whose levels are reduced due to stress and depression.
Stress hormones such as cortisol, a glucocorticosteroid produced by the adrenal cortex, can also act as negative regulators of neuroplasticity. It is well known that the majority of antidepressants used (for chronic use) are capable of increasing levels of BDNF and, apparently, this is part of their therapeutic effect.
Properties of brain neurotrophic factor (BDNF *) and the prospect of its use as an antidepressant

Fig.13. Castrén E., Rantamäki T. The role of BDNF and its antidepressant drug action: Reactivation of developmental plasticity // Developmental Neurobiology. 2010. 70 (5). P. 289–97.
* BDNF plays an important role in many psychopathologies, including and depressions. The use of BDNF itself is impossible due to a number of reasons (which are listed in the figure).
In addition to antidepressants, there are other factors that contribute to an increase in BDNF levels in the CNS, and they coincide with the positive stimuli of neuroplasticity - training, exercise, new experience, diet, etc. Moreover, these factors can often complement drug therapy for depressive disorders.
Figure 14 presents data on the study of the antidepressant properties of BDNF itself in animal models (rats). Since BDNF itself cannot penetrate into the brain (through the blood-brain barrier) with peripheral administration, in the BDNF experiments it was injected directly into the brain.
Study of the antidepressant properties of brain neurotrophic factor (BDNF) in animal models

Fig.14 Eisch AJ, Bolaños CA, de Wit J. et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression // Biological Psychiatry. 2003. 54 (10). P. 994–1005; Shirayama Y., Chen AC, Nakagawa S., Russell DS, Duman RS Brain-derived neurotrophic factor produces antidepressant effects on behavioral models of depression. Journal of Neuroscience. 2002. 22 (8). P. 3251–61.
1. Introduction to the hippocampus . The main idea was to address the introduction of BDNF into the brain area responsible for neurogenesis (the dentate gyrus of the hippocampus is one of the so-called “neurogenic niches” ). After administration, depressive-like behavior of animals was evaluated. (The dysphoric component of depression is evaluated. Animals (rats or mice) are placed in a cylinder with water, from which it is impossible to get out on their own. After some time, the animal's active attempts to get out of the cylinder are replaced by a “state of despair” (the animal is in the water with almost no movement.)
Reduction of immobility (immobilization) of an animal is considered as a correlate of antidepressant effect. BDNF had an antidepressant effect after injection into the dentate gyrus (neurogenic niche) and CA3 zone of the hippocampus (the neurons of this zone ensure the interaction of the dentate gyrus with other areas of the hippocampus).
2. With the introduction of BDNF in the ventral region of the tire (the zone responsible for the production of dopamine and suffering from depressive disorders), the opposite effect was recorded - an increase in depressive-like behavior.
Mimetics
Since it is not possible to use BDNF itself as a medicine, a drug is developed based on this factor. In particular, the active parts of the BDNF molecule (whose spatial structure is caused by the name of the loop, Fig. 15) have been well studied.
Currently, mimetics (activity imitators) of BDNF are being actively studied.
Development of new-generation drugs based on brain neurotrophic factor (BDNF)

Fig.15. Fletcher JM, Morton CJ, Zwar RA et al. Design of a conformationally defined and proteolytically stable circular mimetic of brain-derived neurotrophic factor // The Journal of Biological Chemistry. 2008. 283 (48). P. 33375–83. Massa SM, Yang T., Xie Y. et al. TrkB signaling and prevent small neuronal degeneration in rodents // The Journal of Clinical Investigation. 2010. 120 (5). P. 1774–85. Seredenin S.B., Voronina T.A., Gudasheva T.A. et al. Antidepressive effect of the original low molecular weight mimetic BDNF, dimeric dipeptide GSB-106 // Acta Naturae. 2013. 4 (19). P. 116–120.
Antidepressant properties of peptide mimetics 4 loops of brain neurotrophic factor (BDNF) - GSB-106 compound

Fig. 16. Seredenin SB, Voronina T.A., Gudasheva T.A. et al. Antidepressive effect of the original low molecular weight mimetic BDNF, dimeric dipeptide GSB-106 // Acta Naturae. 2013. 4 (19). P. 116–120.
GSB-106 - a substance of peptide structure, is a mimetic of 4 BDNF loops (domestic development). The substance has an antidepressant effect on animal models with different modes of administration. Currently, extensive research is being conducted on the pharmacological properties of this compound in order to create on its basis an antidepressant of a new generation.
Neurogenesis * and depression
* Neurogenesis is a multistep process of formation of new nerve cells in the mature CNS, which is an adaptive function of the nervous system.

Fuchs E., Flügge G. Adult Neuroplasticity: More Than 40 Years of Research // Neural Plasticity. 2014. Article ID 541870, doi: 10.1155 / 2014/541870

Fig. 17
In Figure 17 we show the neurogenic zones (niches) of mammals:
1) the dentate gyrus of the hippocampus
2) Olfactory bulbs
3) Subventricular zone
It is believed that in humans the main neurogenic niche is the hippocampus (dentate gyrus).
Stress, which is considered as one of the main triggering incentives for depressive disorders, leads to a decrease in BDNF levels and an increase in cortisol, which, in turn, enhances the effect of glutamate on the central nervous system.

Fig. 18
These changes, in aggregate, suppress hippocampal neurogenesis and lead to a decrease in the volume of the hippocampus. Under the action of glutamate, activation of apoptosis (programmed nerve cell death) is also possible. When neurogenesis is impaired, the brain cannot fully compensate for the losses and depressive symptoms develop.
Glutamate is one of the main excitatory amino acids of the CNS. Violation of neuroplasticity under the influence of excessive glutamate action, apparently, is associated with a compensatory reaction. Neurons "remove" unnecessary connections and die (apoptosis) in order to protect the central nervous system from over-stimulation and subsequent damaging effects of this process.
A well-known fact is the ability of antidepressants to stimulate neurogenesis, but the mechanisms underlying this phenomenon are not fully understood to date. It is known that all antidepressant groups act on the monoamine system of the brain and compensate for functional or material deficiencies of serotonin and norepinephrine. In addition, drugs of this pharmacological group increase the levels of brain neurotrophic factor.
Antidepressants can stimulate neurogenesis

Fig. nineteen
Stimulation of neurogenesis is characteristic of antidepressants, regardless of their mechanism of action, chemical structure or class. Consequently, the search for the mechanisms of regulation of neurogenesis should be carried out in common for all antidepressants properties. Such common properties are the activity of antidepressants against serotonin and norepinephrine.
To date, an understanding of the role of serotonin in the regulation of hippocampal neurogenesis is being formed.
Possible mechanisms of neurogenic activity of antidepressants

Fig. 20. Alenina N., Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behavioural Brain Research. 2015. 277. P. 49–57.
First, the dentate gyrus of the hippocampus receives serotonin regulation from large suture nuclei (an accumulation of nerve cells that produce serotonin; are located in the brain stem), both directly and through intercalated neurons, which carry various subtypes of serotonin receptors on their surface.
Secondly, serotonin receptors 1A of the subtype were found on the stem cells themselves , which indicates the potential of serotonin to regulate brain stem cells.
Mechanisms of neurogenic activity of antidepressants

Fig. 21
As shown in experimental studies (in animals and cell cultures), selective serotonin reuptake inhibitors (SSRIs, the classic drug from this group, fluoxetine) are able to stimulate the proliferation stage of neurogenesis in the hippocampus.
The proposed mechanism is an increase in the concentration of serotonin (5-NT - serotonin, also known as 5-hydroxytryptamine) in the central nervous system and the subsequent (enhanced) stimulation of serotonin by neurogenesis. Serotonin receptors of the 1A subtype (5HT1A receptors) may also serve as
potential targets of antidepressants during neurogenesis. These assumptions are consistent with the data on the positive (therapeutic) effect of serotonin 5-HT1A receptor activation by antidepressants (for example, Vilazodone) against depressive disorders.
Another argument that allows us to consider the stimulation of neurogenesis as the main mechanism of action of antidepressants is the coincidence in time between the average time of onset of the therapeutic effect (from 2 to 7 weeks) and the full cycle of neurogenesis (3-7 weeks).
In addition to these mechanisms, antidepressants from the SSRI group also demonstrate the ability to increase levels of BDNF, but the mechanisms of this effect remain unknown.
Effect of fluoxetine (prozac) on the neurogenesis of nonhuman-like primates

Fig. 22. Perera TD, Dwork AJ, Keegan KA, et al. Nonhuman Primates // PLoS ONE. Necessity of Hippocampal Neurogenesis for the Adult Nonhuman Primates // PLoS ONE. 2011. 6 (4): e17600. doi: 10.1371 / journal.pone.0017600.
Studies on primates (the most relevant animal model) showed the ability of fluoxetine (torg. Prozac) to stimulate neurogenesis under stress (in this case, the model of insulating stress was used). Figure 22 shows that fluoxetine significantly increased (statistically significantly) the rate of proliferation (division) of stem nerve cells in the hippocampus of primates.
Effect of fluoxetine on the neurogenesis of nonhuman primates

Fig. 23. Perera TD, Dwork AJ, Keegan KA, et al. Nonhuman Primates // PLoS ONE. Necessity of Hippocampal Neurogenesis for the Adult Nonhuman Primates // PLoS ONE. 2011. 6 (4): e17600. doi: 10.1371 / journal.pone.0017600.
The impact of stress led to a decrease in the granular layer of the dentate gyrus of the hippocampus - the main neurogenic zone of the primate's brain. The introduction of fluoxetine against a stressful exposure prevented this change and maintained normal volume of this structure (total volume).
Effect of fluoxetine on the neurogenesis of nonhuman primates
Correlation between anhedonia (depressive-like behavior) and neurogenesis

Perera TD, Dwork AJ, Keegan KA, et al. Necessity of Hippocampal Neurogenesis for the Non-Primary. PLoS ONE. 2011. 6 (4): e17600. doi: 10.1371 / journal.pone.0017600.
A correlation was found between the stimulation of neurogenesis with fluoxetine and the prevention of the development of depressive behavior (anhedonia).
When studying the effect of fluoxitin on the neurogenesis of nonhuman primates, a significant correlation was established between a decrease in neurogenesis (caused by stress) and an increase in depressive-like behavior in primates (anhedonia, which was determined by the combination of social and eating disorders). -like behavior.
Norepinephrine is another important monoamine that, along with serotonin, is able to participate in the regulation of hippocampal neurogenesis.
Possible mechanisms of neurogenic activity of antidepressants

Fig.25. Jhaveri DJ, Mackay EW, Hamlin AS, et al. Norepinephrine directly activates adult hippocampal precursors via the β3 adrenergic receptors // The Journal of Neuroscience. 2010. 30 (7). H. 2795–2806. doi: 10.1523 / JNEUROSCI.3780–09.2010.
Studies on hippocampal neuron cultures have shown that norepinephrine (unlike serotonin) increased the number of stem cells. Serotonin, as demonstrated earlier, did not affect the quantity, but the rate of proliferation.
In addition to quantitative changes, norepinephrine caused and qualitative - increased the size of the neurospheres, which is clearly shown in the electron microscope image (see fig.26).
Possible mechanisms of neurogenic activity of antidepressants

Fig. 26. Jhaveri DJ, Mackay EW, Hamlin AS, et al. Norepinephrine directly activates adult hippocampal precursors via the β3 adrenergic receptors // The Journal of Neuroscience. 2010. 30 (7). H. 2795–2806. doi: 10.1523 / JNEUROSCI.3780–09.2010.
The role of glial cells in the formation of depressive disorders
Earlier, we considered changes in neurons, but ignored the role of glial cells in the formation of depressive disorders. However, experimental and clinical studies indicate the possible role of the pathology of glial cells in the pathogenesis of depression.
The role of glia in the formation of depressive disorders

Fig. 27. Rajkowska G., Miguel-Hidalgo JJ Gliogenesis and Glial Pathology in Depression // CNS & Neurological Disorders Drug Targets. 2007. 6 (3). P. 219–233.
The researchers presented a scheme for the pathogenesis of depressive disorders with the participation of glia (Fig. 27).
Genetic predisposition may include: a hereditary factor (the presence of a depressive disorder in one of the parents increases the risk of this disease in a child); gene polymorphism: BDNF, a serotonin carrier (which is involved in the reuptake of serotonin into nerve and glial cells), serotonin receptors, serotonin synthesis enzymes (tryptophan hydroxylase type 2).
Genetic vulnerability in combination with environmental and stress factors creates a favorable environment for the formation of depressive disorder.
Scientists have established that the role of glial cells is not the same in young and elderly patients (Fig. 28). Glial cells may play an important role in the pathogenesis of the early stages of depressive disorders, which can cause a marked decrease in the number of pyramidal neurons in more mature age.
The role of glial cells in the formation of depressive disorders varies in young and elderly patients

Fig. 28. Rajkowska G., Miguel-Hidalgo JJ Gliogenesis and Glial Pathology in Depression // CNS & Neurological Disorders Drug Targets. 2007. 6 (3). P. 219–233.
In particular, the loss of function of glial cells at a young age increases the risk of repeated episodes of depression in the elderly, but the deficit of pyramidal neurons rather than glial cells will prevail.

Fig. 29. Rajkowska G., Miguel-Hidalgo JJ Gliogenesis and Glial Pathology in Depression // CNS & Neurological Disorders Drug Targets. 2007. 6 (3). P. 219–233.
Such a dynamics is consistent with one of the most important functions of glial cells - the absorption of an excess of glutamate from the synaptic cleft (contact between nerve cells). Glutamate is one of the main excitatory neurotransmitters of the CNS and its excessive action can lead to disruption of neuroplasticity and excitotoxicity (neurotoxicity associated with excessive excitation; apparently, a protective reaction of nerve cells from overexcitation - the number of neurons and connections between them decreases).
In glial cells there is a protein transporter, which is involved in the transfer of glutamate from the synaptic cleft into the glial cell, where glutamate is metabolized.

Fig. 30. Rajkowska G., Miguel-Hidalgo JJ Gliogenesis and Glial Pathology in Depression // CNS & Neurological Disorders Drug Targets. 2007. 6 (3). P. 219–233.
The impact of stress in combination with other factors leads to:
1. A decrease in the number of glial cells in the structures of the limbic system of the brain;
2. Hyperproduction of glutamate.
In this way, an excess of this excitatory neurotransmitter is formed, which is a negative modulator of neuroplasticity (as it may be part of a compensatory reaction that protects the nervous system from over-stimulation).
The function of glial cells is not limited to the absorption of glutamate, they are also involved in the production of neurotrophins, in particular BDNF (Fig. 31).

Fig. 31
Taken together, a deficit of neurotrophins and an excess of glutamate lead to impaired neuroplasticity and depressive-like changes (decrease in the volume of the hippocampus and the prefrontal cortex, disruption of the normal functional connection between the structures of the limbic circle).
In the context of this concept, it is also possible to find explanations for the therapeutic efficacy of antidepressants (Fig. 32):
1. Antidepressants can “soften” the effect of stress by normalizing the activity of the hypothalamic-pituitary-adrenal axis;
2. Increase the concentration of BDNF in the central nervous system;
3. Stimulate the processes of neuroplasticity.

Fig. 32
Figure 33 presents a generalized depressive disorder scheme that is based on the concept of stress-mediated neurodegeneration. It is seen that antidepressants occupy a niche "proofreaders of the effects of stress." With all the advantages and therapeutic potential available, antidepressants are not always effective in eliminating depressive symptoms.

Fig. 33
There are so-called pharmacotherapy-resistant forms of depressive disorders. This phenomenon is attempted to be explained both by the diversity of stress factors, and by the different strength and duration of stress exposure, and by individual characteristics (mutation of targets of antidepressants). But the general conclusion from this situation is one - the search for fundamentally new targets for the pharmacotherapy of depressive disorders is needed.
New trends in the creation of antidepressants
A promising direction is the impact on the system of glutamate, if we consider this neurotransmitter as one of the key elements of the pathogenesis of depressive disorders. Within this area, significant success has been achieved - a fundamentally new antidepressant has been created, which, by its mechanism, is a glutamate blocker for NMDA receptors and prevents the excessive activity of this amino acid. Rapasintel antidepressant has now successfully completed phase I and phase II clinical trials, where it has been shown to be highly effective and is considered as a treatment for resistant forms of depressive disorders.
In the framework of the glutamatergic theory of depressive disorders, the role of the main inhibitory mediator of the central nervous system - gamma-aminobutyric acid (GABA or GABA) can be considered.

Fig. 34. Möhler H. The GABA system in anxiety and its therapeutic potential // Neuropharmacology. 2012. Jan. 62 (1). P. 42–53.
GABA is the functional opposite of glutamate and is capable of limiting its stimulating effect; therefore, an assessment of the role of GABA in depressive disorders appears to be quite logical.
It has been established that, against the background of depressive disorders, there is a shortage of cortical levels of GABA and its receptors. In particular, pyramidal neurons that produce glutamate can be inhibited by the inhibitory effects of interneurons that produce GABA. GABA realizes its inhibitory action through the activation of the GABA-A receptor.

Fig. 35. Möhler H. The GABA system in anxiety and its therapeutic potential // Neuropharmacology. 2012. Jan. 62 (1). P. 42–53.
The structure of the GABA-A receptor is shown in Figure 35. The receptor consists of 5 subunits (2α, 2β and γ), each subunit has a subtype, for example, 6 variants of α-subunits are known. The combination of different subunit variants determines the GABA-A receptor subtype.
In confirmation of the role of GABA, the effectiveness of the positive modulator of the GABA-A receptor, eszopiclone, also speaks. The target of this drug is GABA-A receptors, carrying in its composition the α2- and α3-subunits. Eszopiclonesometimes used in combination with antidepressants and significantly relieves depressive symptoms even after the withdrawal of antidepressants. It is believed that its therapeutic effect is associated with a weakening of the function of glutamate. Interestingly, other positive modulators of the GABA-A receptor (for which other variants of α subunits, such as zolpidem , are necessary ) do not possess this activity.

Fig. 36
And so, in the presented scheme (Fig. 37) we summarize the data on the impairment of the processes of neuroplasticity and the development of depressive symptoms.
1) The suppression of neuroplasticity processes is not strictly specific for depressive disorders, but is also observed in other psychopathologies (schizophrenia, bipolar affective disorder) and neurological diseases (multiple sclerosis, Parkinson's disease, Alzheimer's disease).
2) In animal models, the suppression of stem cell proliferation by chemical agents does not block the effects of antidepressants (unlike x-rays, where the opposite effect is noted).
3) Resource neurogenesis may be limited, and excessive stimulation can lead to depletion.
4) The long-term effects of prolonged artificial stimulation of neurogenesis are unknown. Is there a risk of developing a tumor?
5) Disruption of neuroplasticity is not an exhaustive concept of depressive disorders. The concept cannot fully explain the presence of ALL symptoms of the disease (for example, somatization of depressive symptoms, when symptoms of depression are masked as diseases of internal organs - headache, pain in the heart, abdomen, etc.), nature of the course (cyclical) and resistance some forms of depression to drug therapy (despite the fact that antidepressants activate neurogenesis and increase BDNF).
Disruption of neuroplasticity and depressive disorders - concept flaws

Fig. 37
- Suppression of neuroplasticity processes is not specific for depressive disorders.
- In animal models, suppression of stem cell proliferation does not always block the effects of antidepressants.
- Resource neurogenesis may be limited, and excessive stimulation can lead to depletion.
- The long-term effects of long-term "violent" stimulation of neurogenesis are unknown. Is there a risk of developing a tumor?
- Violation of neuroplasticity is not an exhaustive concept of depressive disorders.
Theories about the role of neurotransmitter amino acids - glutamate and GABA are not exhaustive. First, the presented systems (glutamatergic and GABAergic) cannot be considered in isolation from other factors, since in fact, they are an intermediate in the pathogenesis of depressive disorders or individual symptoms. Secondly, changes in the glutamatergic and GABAergic systems of the brain are observed not only in depressive, but also in a number of other disorders and conditions (schizophrenia, anxiety disorders, panic attacks, epilepsy, pain sensitivity, Parkinson's disease, Alzheimer's disease).

Fig. 38
If we consider pharmacological intervention in the glutamatergic system, then it is undoubtedly promising and even innovative, since previously, all antidepressants acted solely on the monoaminegic system of the brain. However, such a wide functional of glutamate in the central nervous system can lead to the development of undesirable effects and a number of contraindications. But it is too early to make predictions; the blocker of glutamate receptors has yet to go through phase III of clinical trials. The drug is not considered as a replacement for modern antidepressants, but as a means of complementary therapy (for example, in case of stable forms of depression).
Drugs, which are positive modulators of GABA-receptors, are not currently regarded as independent antidepressants, they are useful in eliminating certain symptoms of depressive disorders.
"Perfect" drug
A promising direction for the development of new effective and safe antidepressants is the study of the mechanisms of stress itself, since it is the stress factor (stressful events) that is considered as the main starting stimulus for the formation of depressive disorders.
What should a promising drug look like?
The search and creation of the “ideal” anti-depressant should be based on two simple principles (according to Franco Borsini):
1. The drug should not change the psyche of a healthy person.
2. The drug should act only in terms of psychopathology.

Fig. 39. Future directions // World Journal of Pharmacology. 2012. 1 (1). P. 21–29.
Interrupting the mechanisms of stress in the early stages would prevent all those changes that were considered in the context of our article. This method of correction, in theory, seems to be the most effective not only to prevent the development of depression, but also to provide reliable protection against relapses and repeated, more severe episodes.
Drug therapy, by itself, is not the only means of correcting the psychopathology in question. No less importance should be given to communication with patients, identifying the cause of the disease. In some cases, the effectiveness of psychotherapy also has a high potential, because helps to find solutions to the problem, rather than struggling with the symptoms of the disease, leaving the problem unresolved and translating depression into subchronic and chronic forms.
All Articles