The FDA approved the first "direct" gene therapy for the treatment of genetic diseases.
The gene therapy drug Luxturna, developed by Spark Therapeutics, helps restore vision in patients with an inherited form of blindness.
A dose of viruses filled with DNA, administered directly into the eyes, is the first gene therapy allowed in the United States to treat a genetic disease. The resolution of such a drug is a significant moment for the region riddled with ups and downs, since the first study on gene therapy began almost three decades ago.
Luxturna is the third gene therapy approved this year. In August, the agency approved Kymriah from Novartis (CAR-T-cell immunotherapy, in which the patient's immune cells are removed, modified outside the body, and then returned to the bloodstream to fight leukemia). The second approved CAR-T cell immunotherapy was Yescarta from Gilead Sciences. In contrast to the above, Luxturna is the first drug that corresponds to the “traditional” perception of gene therapy, when viruses filled with DNA are introduced directly into the body. To date, more than 600 clinical trials using gene therapy are being conducted in the United States.
The origins of Luxturna began with Jean Bennett research at the University of Pennsylvania in the 1990s. In 2000, she tested gene therapy to treat genetic retinal diseases in dogs. However, there were difficulties with further funding after wide publicity of patient deaths in other clinical trials of gene therapy. Bennett later collaborated with the Children's Hospital of Philadelphia, which decided to commercialize the technology, providing $ 50 million to launch Spark Therapeutics in 2013.
Luxturna improves vision in people with a mutation in the RPE65 gene, which encodes an enzyme that is important for vision.
Exposure to light leads to the transition of “compressed” cis-retinal to “compressed” trans-retinal. This physical change triggers the transmission of a signal to the brain. To restart the cycle, the RPE65 enzyme returns the trans-retinal back to cis-retinal. However, people who have a genetic mutation that impede the production of RPE65 cannot “reset the cycle”. This leads to visual impairment, which usually begins in infancy or in childhood and leads to almost complete blindness.
Regeneration of photosensitive cells with the normally working RPE65 enzyme (phototransduction of trans-retinol to 11-cis-retinal - wiki ):
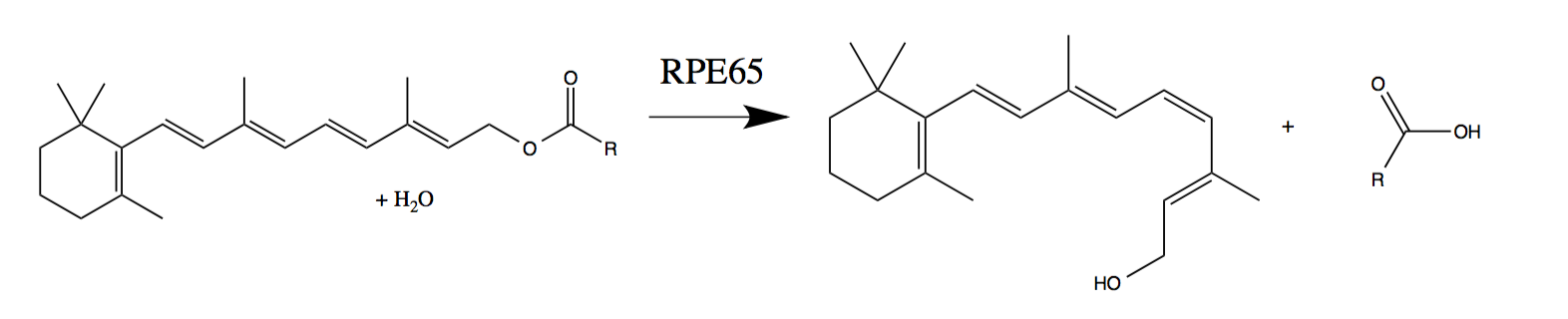
Although many mutations in RPE65 can lead to blindness, Luxturna should treat them all in theory. The tool contains a complete copy of RPE65 packed into an adeno-associated virus, necessary for transferring DNA into retinal cells in order to obtain a fully functional enzyme.
The tool is intended for a single therapy, when the surgeon injects a solution containing about 150 billion viral particles filled with DNA, directly under the retina of each eye. Tests on obstacles with different levels of illumination showed that the treatment improved the vision of the patients.
According to the FDA, there are 1,000–2,000 patients in the United States with RPE65 mutations that may benefit from Luxturna. However, the high price of the drug may be a problem for getting therapy even for this small number of patients. The price has not yet been disclosed, but according to Spark’s CEO, the cost of therapy may exceed $ 1 million per patient.
Supplement, old video with an animated demonstration of drug administration:
Still
What can people see with this form of blindness:
Jean-Bennett and a demonstration of the success of therapy on the example of a child with RPE65 problems:
Jean-Bennett and a demonstration of the success of therapy on the example of a child with RPE65 problems:
All Articles