Revision of the SI system of units: new definitions of ampere, kilogram, Kelvin and mole
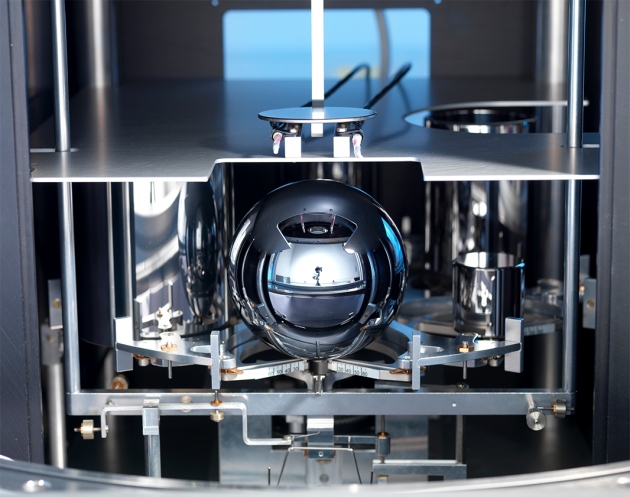
A silicon-28 sphere with a purity of 99.9998% can be used to calculate the most accurate Avogadro number, which will be included in the definition of the unit for measuring the amount of a substance, known as a mole. Photo: UK National Physical Laboratory
The International Bureau of Weights and Measures plans to carry out the most significant reform in the international system of units (SI) since the last major revision of this standard in 1960, Nature writes . We will have to accept new GOSTs, as well as make corrections to the textbooks of physics in schools and universities.
Currently, SI (the modern version of the metric system) is accepted as the basic system of units by most countries of the world and is used almost everywhere in the field of technology. The full definition of all SI units is given in the official brochure (8th edition) and its addendum of 2014 . The current standard was approved in the USSR on January 1, 1963 by GOST 9867-61 "International System of Units".
The leadership of the international organization will vote for the proposed changes at the General Conference on Weights and Measures in 2018 , and in case of a positive decision, the changes will take effect from May 2019. New definitions for units of measurement and standards will not affect the lives of ordinary people: one kilogram of potatoes in the store will remain the same kilogram of potatoes. Scales will measure vegetables and meat with the same accuracy as before. But these definitions are important for scientists, because in scientific research the perfect accuracy of formulations and measurements must be respected. The International Bureau of Weights and Measures believes that new standards will allow "to ensure the highest level of accuracy in various measurement methods at any place and time and on any scale, without loss of accuracy."
So, what changes are waiting for us?
Now the International Bureau of Weights and Measures intends to revise the definitions and standards of the following units of measurement:
- ampere
- kilogram
- kelvin
- mole
It should be noted that hereinafter the new definitions are given in abbreviated form and do not correspond exactly to the text, which is recorded in an official document. The document itself and the final values of the constants will be published soon.
Kilogram
The modern definition was adopted by the III General Conference on Weights and Measures (GKMV) in 1901 and is formulated as follows: "A kilogram is a unit of mass equal to the mass of the international prototype kilogram." At the same time, the International prototype (standard) of a kilogram is stored at the International Bureau of Weights and Measures (located in the city of Sevres near Paris) and is a cylinder with a diameter and height of 39.17 mm from a platinum-iridium alloy (90% platinum, 10% iridium). The size of the prototype roughly corresponds to the size of a golf ball.
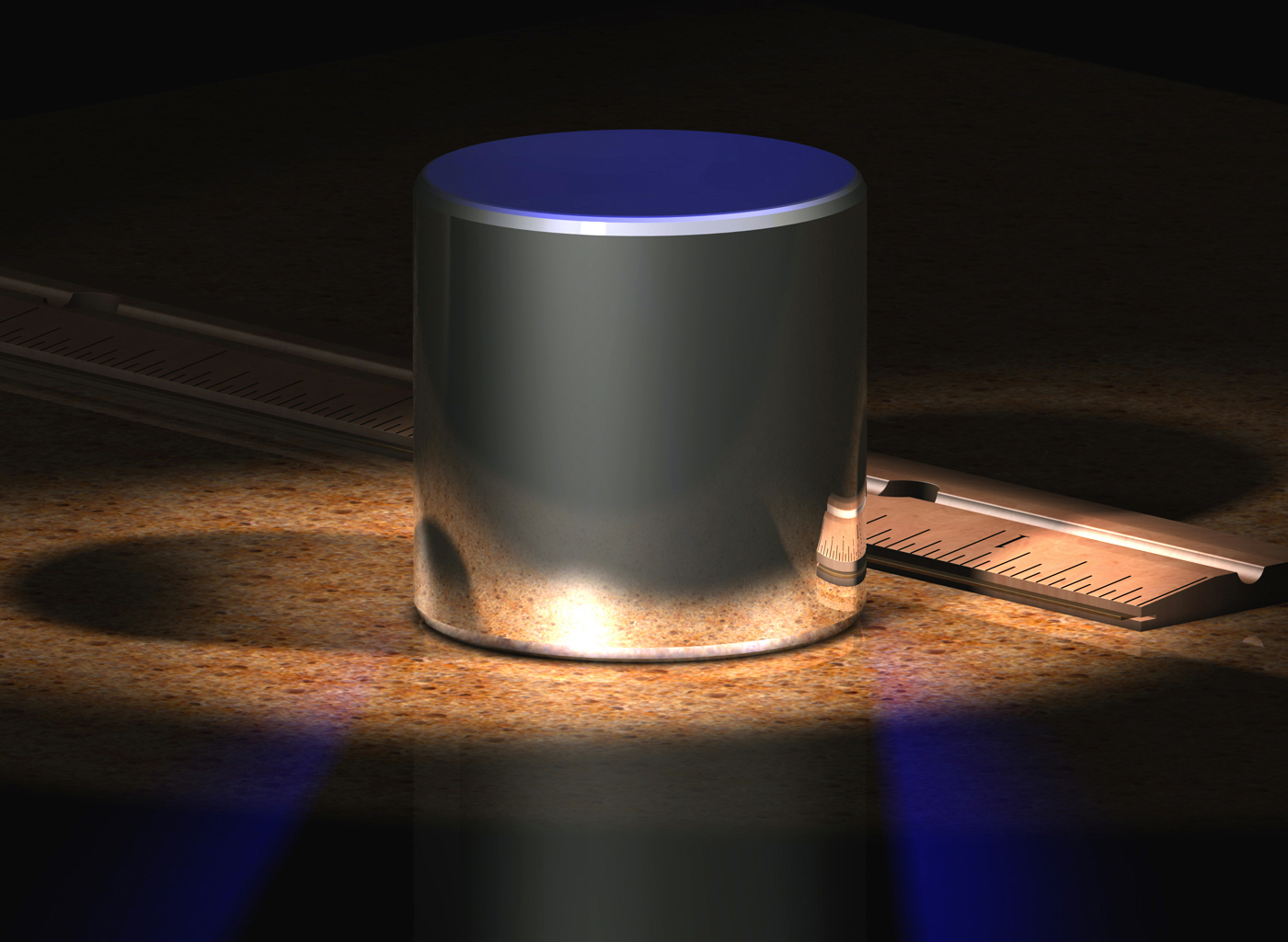
Computer image of the international kilogram prototype
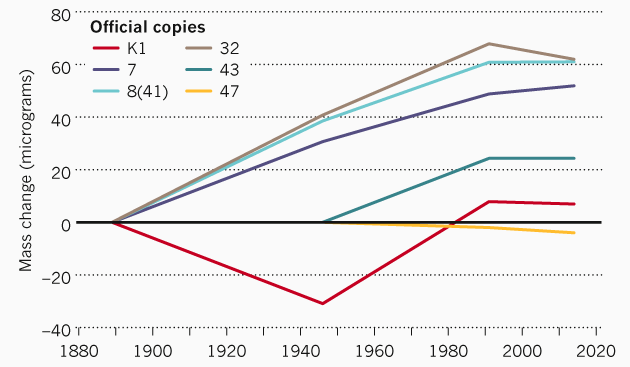
The problem with the kilogram standard is that any materials can lose atoms or, on the contrary, replenish with atoms from the surrounding space. In particular, various official copies of the reference kilogram, which is stored in Sevres, differ in weight from the official reference. The difference reaches 60 micrograms. Such changes have occurred in more than 100 years since the creation of copies.
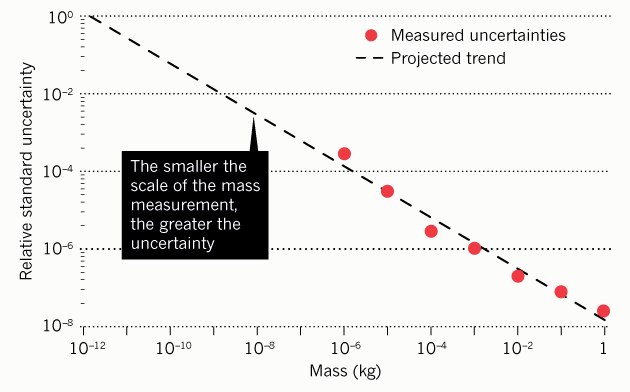
Another problem with units of measurement of a fixed scale is that the element of uncertainty (error) increases with distance from this fixed point (reference). For example, now when measuring a milligram, the element of uncertainty is 2500 times larger than when measuring a kilogram.
This problem is solved by determining the unit of measure through another physical constant. Actually, in the new definition of the kilogram this is done: the Planck constant is used here.
The new definition : 1 kilogram is Planck's constant divided by 6.626070040 × 10 −34 m 2 · s −1 . To express one, Planck's constant is required.
Measurement of mass in practice is possible with the help of watt weights : through two separate experiments with comparison of mechanical and electromagnetic force, and then by moving the coil through a magnetic field to create a potential difference (in the illustration below). Roughly speaking, the mass is calculated through the electricity, which is necessary to lift the object lying on the other side of the scale.
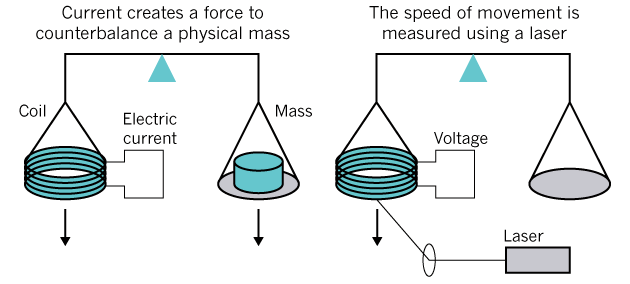
Kelvin
Modern definition : as written in GOST, 1 Kelvin equals 1 / 273.16 of the thermodynamic temperature of the triple point of water. The beginning of the scale (0 K) coincides with the absolute zero. In the obligatory Technical Annex to the text of the ITS-90 International Temperature Scale, the Consultative Committee on Thermometry set the requirements for the isotopic composition of water when the temperature of the triple point of water is realized.
The triple point of water is strictly defined values of temperature and pressure, at which water can simultaneously and equilibrium exist in the form of three phases - in solid, liquid and gaseous states.
The International Committee for Weights and Measures has confirmed that the definition of Kelvin refers to water, whose isotopic composition is determined by the following relations:
0.00015576 mol 2 H per mole 1 N
0.0003799 mol 17 O for one mole 16 O
0,0020052 mole 18 O for one mole of 16 O.
The problems of modern definition are obvious. In the practical implementation of the magnitude, Kelvin depends on the isotopic composition of water, but in practice it is almost impossible to achieve the molecular composition of water, which corresponds to the Technical Annex to the text of the ITS ‑ 90 International Temperature Scale.
Back in 2011, at a meeting of the General Conference on Weights and Measures, it was proposed to redefine kelvin in a future edition of the International System of Units, associating it with the value of the Boltzmann constant. Thus, the value of Kelvin will be accurately recorded for the first time.
The new definition : 1 Kelvin corresponds to a change in thermal energy of 1.38064852 × 10 −23 joules. The expression of the unit requires the Boltzmann constant.
You can measure the exact temperature by measuring the speed of sound in a sphere filled with gas. The speed of sound is proportional to the speed of movement of atoms.
Moth
The modern definition : a mole is the amount of a substance in a system containing as many structural elements as there are atoms in carbon-12 and a mass of 0.012 kg. When using moles, structural elements must be specified and can be atoms, molecules, ions, electrons and other particles or specified groups of particles.
A new definition : the amount of substance of a system that contains 6.022140857 × 10 23 specified structural units. To express one, Avogadro constant (Avogadro number) is required.
To calculate the Avogadro number — and determining the mole through it — scientists suggest creating an ideal sphere of pure silicon-28. This substance has an ideally accurate crystal lattice, so that the number of atoms in a sphere can be determined by accurately measuring the diameter of the sphere (using a laser system). In contrast to the existing piece of platinum-iridium alloy, the rate of loss of silicon-28 atoms is precisely predictable, which allows you to make adjustments to the standard.
The first experiments on the creation of such a standard were undertaken in 2007. Researchers from the Berlin Crystal Growing Institute under the direction of Helge Riemann purchased in Russia silicon-28 enriched silicon and managed to obtain an isotope sample 28 with a purity of 99.994%. After that, the researchers analyzed the composition of 0.006% "extra" atoms for several years, determined the exact volume of the sphere, and performed an x-ray analysis. Initially it was assumed that the “ideal” spheres of silicon-28 could be approved as a new standard for a kilogram. But now it is more likely that they are used to calculate the Avogadro number, and, as a consequence, the definition of a mole. Moreover, during the time that has passed since 2007, physicists have learned how to produce much cleaner silicon-28.

Sphere from silicon-28 with purity 99,9998. Photo: CSIRO Presicion Optics
In 2014, American physicists were able to enrich silicon-28 to an unprecedented quality of 99.9998% as part of an international project to calculate the Avogadro number.
Ampere
The modern definition was proposed by the International Committee for Weights and Measures in 1946 and adopted by the 9th General Conference on Weights and Measures (GKMV) in 1948: “Amperes is a constant current force that, when passing along two parallel straight conductors of infinite length and a negligibly small area of circular transverse a cross section located in vacuum at a distance of 1 meter one from the other would cause an interaction force of 2 · 10 −7 newton in each conductor section 1 meter long. ”
In the modern definition of an ampere is determined through a kind of thought experiment, which provides for the occurrence of a force in two wires of infinite length. It is obvious that in practice we cannot measure such a force, because, by definition, there cannot exist two conductors of infinite length.
The definition of amperes was proposed at the same meeting of the General Conference on Weights and Measures in October 2011 as the definition of Kelvin. The idea was that a new definition should be based not on man-made artifacts through a thought experiment, but on fundamental physical constants or properties of atoms. So, the new definition is expressed only through one constant - the electron charge.
New definition : electric current corresponding to a flow of 1 / 1.6021766208 × 10 −19 elementary electric charges per second. To express a unit, an electron charge is required.
In practice, to determine the ampere, you need only one instrument - a single-electron pump. Such tools created a few years ago . They allow you to move a certain number of electrons during each pumping cycle, which is an extremely valuable quality for basic science and metrology.
The definitions of second, meter, and candela appear to remain unchanged, as shown in the illustration .
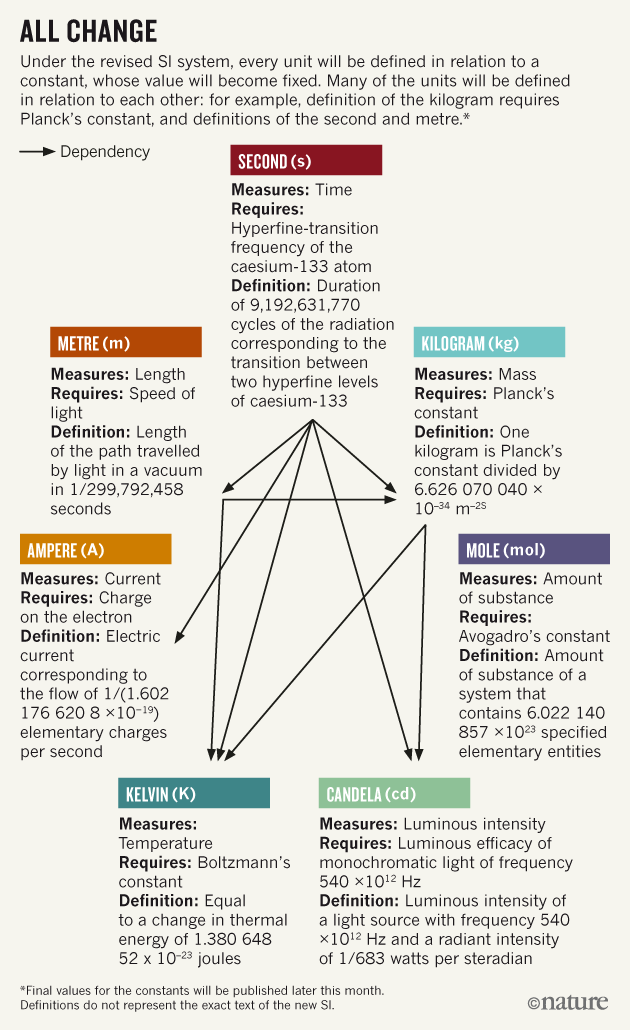
In the new SI system, the definition of all units is expressed in terms of a constant with a fixed value. Many units are defined in conjunction with other units. For example, the definition of a kilogram is determined through Planck's constant, as well as through the definition of seconds and meter.
It is believed that such a system is much more stable and self-sufficient.
All Articles