Guide to electrical materials for all. Part 10
Continuing guidance on electrical materials. In this part we end up with dielectrics: polyimides, polyamides, polymethyl methacrylate, polycarbonate. Even in this part of the picture I killed a lot of time.

Welcome to CAT (TRAFFIC)
(Polyimides - a whole class of polymers, but mostly it is about the Capton)
Heat-resistant flexible transparent polymer of yellow color. Often confused with polyamide, in
the power of consonance. Sometimes appears under the brand name "Kapton". Keeps the temperature
up to + 400 ° , it does not get cold in the cold.
Heat resistant dielectric. The heating element of the glue gun made of ceramic
The rope is wrapped in a Kapton film, to isolate the electrodes from the body.

Connector, flexible cable, amplifier chip - mounted on a polyimide substrate.
Material for the manufacture of flexible printed circuit boards. Often in electronic devices
You can meet flexible printed circuit boards on a yellow transparent plastic, which bend and connect the blocks in the role of a loop, having soldered radio elements along. The substrate of such boards is polyimide.
Another class of polymers. You are probably familiar with polyamide-6 and polyamide-6.6, but
not by chemical name, but by brand - it is capron and nylon.
Polyamides are used extensively, from the shells of some sausages and ending with women's tights.
Capron in the form of rods, sheets, blocks has the name "Kaprolon", it can be antifriction, due to the addition of graphite, molybdenum disulfide. From caprolon, for example,
running gear nuts are made as a cheap alternative to bronze.

Various products from nylon - gears, couplers.
Polyamide with fiberglass filling is a very durable material made from such plastic
make mechanically loaded parts - parts of furniture, gears, body.
Nylon ties are an indispensable thing in organizing wiring harnesses, fast
and secure fastening of everything and everything.
Fibers - ropes, cords, twine, threads. As reinforcing threads in some
cable types.
Running nuts are a cheap replacement for bronze in running nuts of machines and mechanisms.
Other names - plexiglass, plexiglass, acrylic. Transparent fragile plastic. Resistant to
UV (with additives), fuels and lubricants.
Quite a popular material among homemade artists - is cut by laser, milled. Well molded in the heated state, bends. Transparent goods holders on display windows, transparent hemispheres, embossed light boxes are all PMMA.
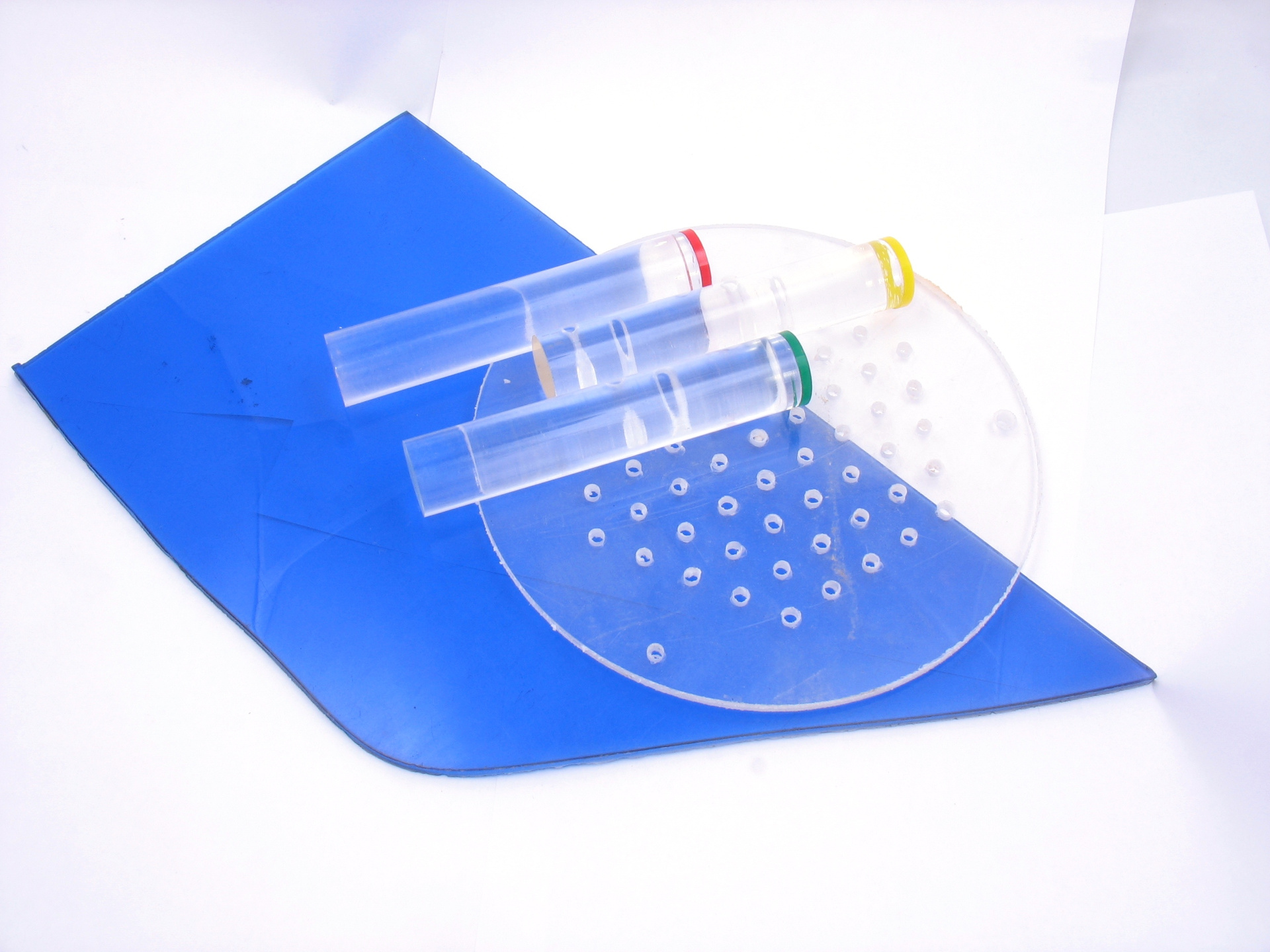
Polymethyl methacrylate is available in both transparent and colored. Rods in the photo are used as light guides.
It dissolves in dichloroethane, which is often mistakenly called “glue for plexiglass,” burst when bent, and does not turn white at the bend. The smell of burning PMMA is not confused with anything.
It is used in various light guides, translucent structures. Low ductility and cracking limits the use of PMMA in applications where impact protection is needed.
Probably the most affordable of transparent polymers, you can buy both in sheets and in rods, blocks. Good glued, polished, processed.
Transparent durable plastic. Unlike PMMA, it has the best toughness, which makes it preferable in tasks where strength is needed, where the polycarbonate will withstand, PMMA will be covered with cracks.
Not resistant to organic solvents, contact with gasoline, oils can cause destruction and the appearance of cracks.

Polycarbonate products - safety glasses and compact disc.
CDs. The transparent base of the disc is polycarbonate. The basis of optical lenses (often covered with protective layers, polycarbonate is easily scratched). Due to the high impact resistance - various protective helmets, masks, visors, safety glasses. Cellular polycarbonate - extruded plastic panels - used in greenhouses.
Without the addition of special additives destroyed in the sun. This can be seen on old
cheap polycarbonate greenhouses.
The graph appeared out of curiosity, it became interesting, from which it was possible to make insulation
wires during the second world war. (It's amazing how much time it took to search and process information for just one picture. But, perhaps, it turned out to be one of a kind.) Searching for information on the Internet and not finding anything, I had to shovel the story for each material separately. On the chart, the line starts in the year when the polymer was presented as a commercial product that is produced in tons and can be bought. The smooth disappearance of the line indicates that the material has lost its popularity and has been supplanted by other materials.
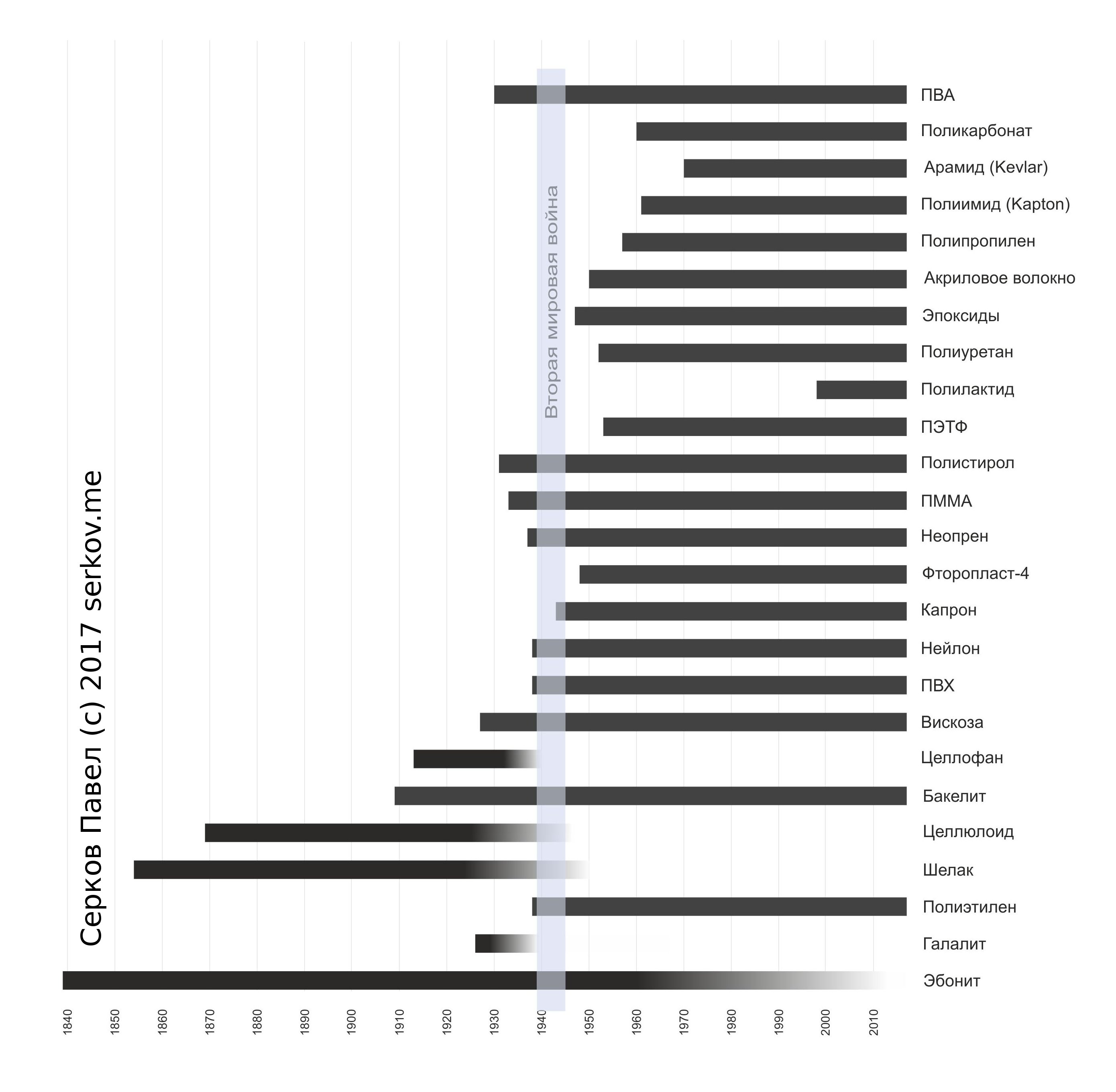
The time between the discovery of material in the laboratory and its mass synthesis at the plant varied from several years (Nylon, Bakelite, PMMA) to dozens of years (Polyethylene, PVC). It's one thing to get a cascade of reactions in the laboratory and from kilograms of raw materials to get one gram of material, and another thing is to establish fast, inexpensive synthesis with a good yield of the product. In addition, manufacturers are faced with the problem of "chicken and eggs": There is no demand for polymer from producers, as there is no factory for the production, and, therefore, reliable supplies. A plant is not built because there is not enough demand for the product.
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final

Welcome to CAT (TRAFFIC)
Polyimide
(Polyimides - a whole class of polymers, but mostly it is about the Capton)
Heat-resistant flexible transparent polymer of yellow color. Often confused with polyamide, in
the power of consonance. Sometimes appears under the brand name "Kapton". Keeps the temperature
up to + 400 ° , it does not get cold in the cold.
Application examples
Heat resistant dielectric. The heating element of the glue gun made of ceramic
The rope is wrapped in a Kapton film, to isolate the electrodes from the body.

Connector, flexible cable, amplifier chip - mounted on a polyimide substrate.
Material for the manufacture of flexible printed circuit boards. Often in electronic devices
You can meet flexible printed circuit boards on a yellow transparent plastic, which bend and connect the blocks in the role of a loop, having soldered radio elements along. The substrate of such boards is polyimide.
Polyamides
Another class of polymers. You are probably familiar with polyamide-6 and polyamide-6.6, but
not by chemical name, but by brand - it is capron and nylon.
Polyamides are used extensively, from the shells of some sausages and ending with women's tights.
Capron in the form of rods, sheets, blocks has the name "Kaprolon", it can be antifriction, due to the addition of graphite, molybdenum disulfide. From caprolon, for example,
running gear nuts are made as a cheap alternative to bronze.

Various products from nylon - gears, couplers.
Polyamide with fiberglass filling is a very durable material made from such plastic
make mechanically loaded parts - parts of furniture, gears, body.
Application examples
Nylon ties are an indispensable thing in organizing wiring harnesses, fast
and secure fastening of everything and everything.
Fibers - ropes, cords, twine, threads. As reinforcing threads in some
cable types.
Running nuts are a cheap replacement for bronze in running nuts of machines and mechanisms.
Polymethyl methacrylate - PMMA
Other names - plexiglass, plexiglass, acrylic. Transparent fragile plastic. Resistant to
UV (with additives), fuels and lubricants.
Quite a popular material among homemade artists - is cut by laser, milled. Well molded in the heated state, bends. Transparent goods holders on display windows, transparent hemispheres, embossed light boxes are all PMMA.

Polymethyl methacrylate is available in both transparent and colored. Rods in the photo are used as light guides.
It dissolves in dichloroethane, which is often mistakenly called “glue for plexiglass,” burst when bent, and does not turn white at the bend. The smell of burning PMMA is not confused with anything.
It is used in various light guides, translucent structures. Low ductility and cracking limits the use of PMMA in applications where impact protection is needed.
Probably the most affordable of transparent polymers, you can buy both in sheets and in rods, blocks. Good glued, polished, processed.
Polycarbonate
Transparent durable plastic. Unlike PMMA, it has the best toughness, which makes it preferable in tasks where strength is needed, where the polycarbonate will withstand, PMMA will be covered with cracks.
Not resistant to organic solvents, contact with gasoline, oils can cause destruction and the appearance of cracks.

Polycarbonate products - safety glasses and compact disc.
Application examples
CDs. The transparent base of the disc is polycarbonate. The basis of optical lenses (often covered with protective layers, polycarbonate is easily scratched). Due to the high impact resistance - various protective helmets, masks, visors, safety glasses. Cellular polycarbonate - extruded plastic panels - used in greenhouses.
disadvantages
Without the addition of special additives destroyed in the sun. This can be seen on old
cheap polycarbonate greenhouses.
Graph of the history of the industrial use of polymers
The graph appeared out of curiosity, it became interesting, from which it was possible to make insulation
wires during the second world war. (It's amazing how much time it took to search and process information for just one picture. But, perhaps, it turned out to be one of a kind.) Searching for information on the Internet and not finding anything, I had to shovel the story for each material separately. On the chart, the line starts in the year when the polymer was presented as a commercial product that is produced in tons and can be bought. The smooth disappearance of the line indicates that the material has lost its popularity and has been supplanted by other materials.

The time between the discovery of material in the laboratory and its mass synthesis at the plant varied from several years (Nylon, Bakelite, PMMA) to dozens of years (Polyethylene, PVC). It's one thing to get a cascade of reactions in the laboratory and from kilograms of raw materials to get one gram of material, and another thing is to establish fast, inexpensive synthesis with a good yield of the product. In addition, manufacturers are faced with the problem of "chicken and eggs": There is no demand for polymer from producers, as there is no factory for the production, and, therefore, reliable supplies. A plant is not built because there is not enough demand for the product.
Links to parts of the manual:
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final
All Articles