Guide to electrical materials for all. Part 2
Continuing guidance on electrical materials. In this part, we continue to disassemble the conductors: Iron, Gold, Nickel, Tungsten, Mercury.

Welcome to CAT (TRAFFIC)
Fe is iron. The main structural material in industry is also used in electrical engineering. Bad, compared to copper, electrical conductivity is compensated by a very low price. And, more importantly in Russia, less attractive for metal hunters, the grounding from a thick rusty pipe will stand without protection longer than a beautiful copper bus.
In the technique of iron is used almost exclusively in the form of alloys with carbon - iron and steel. The properties of steels of different grades are very different: from soft to hard tool.
Hardware. Screws, washers, nuts of steel are made in large quantities on specially designed equipment for this. Hardware from other metals are very rare and much more expensive. Therefore, in most cases, the copper tip of the copper wire will be drawn to the copper bus with a steel bolt. Also important is the high strength of steel, the copper bolt does not tighten with the force of steel. Pay attention to the numbers on the bolt head: they indicate its strength. The larger the number, the more you can tighten the bolt.
Terminal blocks, connectors. The well-known "nuts" contain steel plates with a protective coating against corrosion. Also, the use of steel is necessary to prevent galvanic corrosion when connecting copper and aluminum wires.

Connector "nut". Inside the plastic shell, a set of steel plates with screws allows you to make a branch from the cable core without cutting the core itself. Also allows you to go from aluminum to copper.
Ground loops. Electrical safety requirements require grounding. Often, in industrial conditions, the grounding bus is made of rolled steel, fixed along the perimeter of the wall. Poor electrical conductivity of steel is compensated by a large cross section of the conductor. In many cases, safety regulations and standards prescribe that the grounding parts should be made of steel for reasons of mechanical strength.

Steel strip, the envelope of the column - the ground bus.
Magnetic properties of steel are widely used - transformer cores and chokes are assembled from steel plates.
Corrosion. Iron rust, while the density of rust below the density of the original iron, because of this, the design swells . Therefore, iron is coated with protective coatings - galvanized, tinned, chrome-plated, painted, etc. Different steel grades are subject to corrosion to varying degrees, and according to the law of meanness, it is those that are most easily processed on machines that rust the most.
Au - Gold. The most stupid precious metal. It has the least application in technology of all precious metals, but is a symbol of wealth. Surprisingly more expensive than platinum (2017), which is devoid of common sense and is only the result of speculation.
Contact Covers Due to the fact that gold does not oxidize in air, the contacts are covered with a very thin layer of gold.

Gold coating on various electronic components: coating on the contacts of the board for installation in the slot, coating on the contacts of the membrane buttons of the mobile phone, coating on the pins of the processor.
Corrosion protection. In some demanding applications, a gold coating is used to protect the conductors from corrosion (mainly military). Once the gold coating was the only way to protect electronic components from corrosion in the jungle, so many old radio components have even gilded cases. And now they usually just fill the board with a compound in a “brick”.
Ni - Nickel. A wonderful metal, but in electronic technology the main application - as a cheap alternative to gold - is the coating of contacts. If the contact is coated with white shiny metal, then it is most likely nickel.
Contact plating. It is applied to copper, plastic, for reliable contact and for decorative purposes. Greedy Chinese sometimes make plastic contacts, covering the top with a layer of nickel and chromium, it looks normal, it even works, but it’s not a question of reliability.

Various nickel-plated connectors for reliable contact.
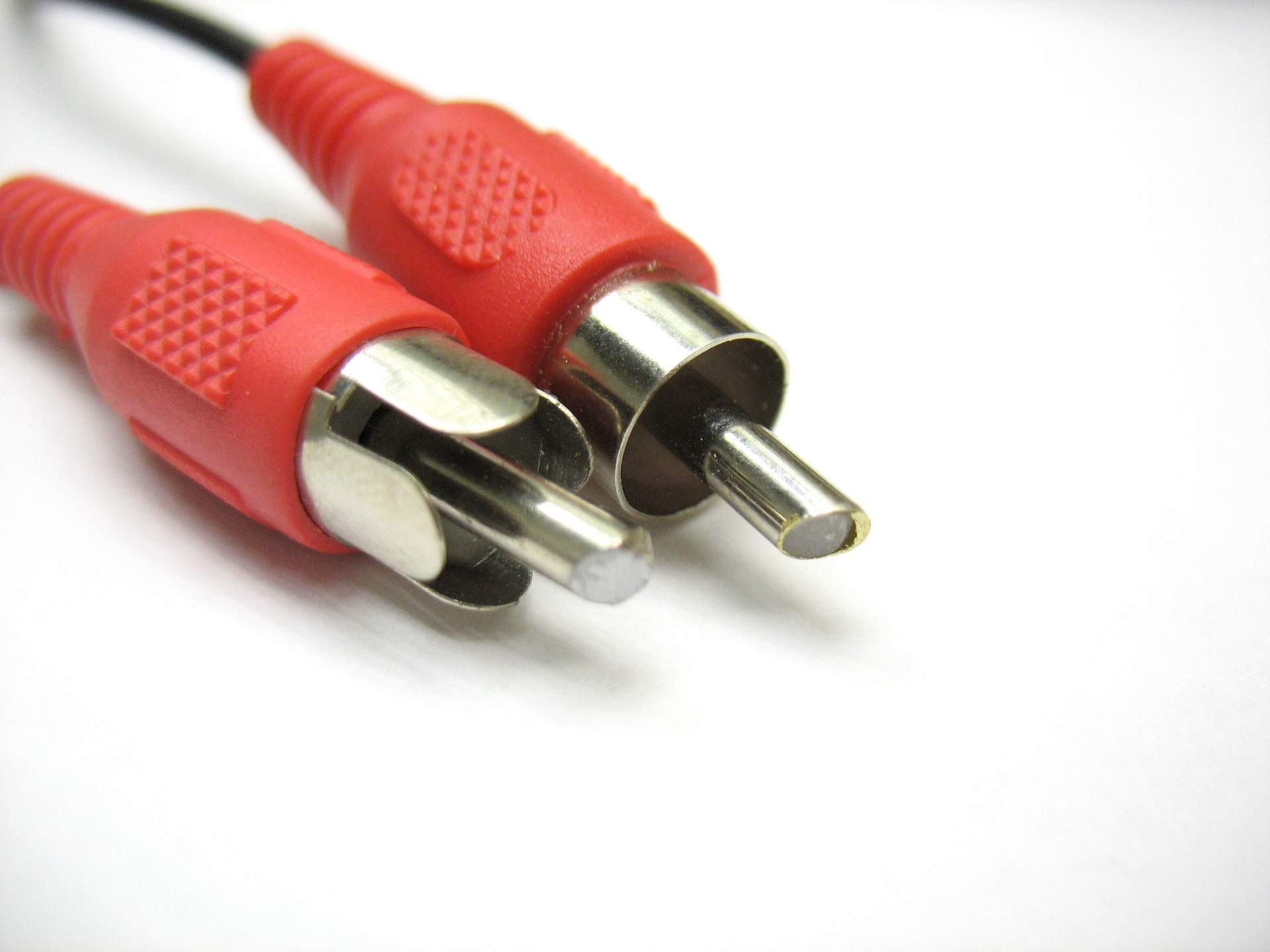
At the connector on the right, to save metal, the core of the pin is made hollow with plastic filling. Brass nickel-plated tubing from which the pin is made, not the worst option.
Tokovody at the lamps. Platinite alloy (46% Ni, 0.15% C, the rest is Fe) does not contain platinum, but has a linear thermal expansion value very close to platinum, which allows it to be made of reliable electrodes passing through glass. When the temperature changes, such electrodes do not cause glass cracking and loss of tightness.
Intermediate protective layers. To protect against corrosion, mutual diffusion of metals when creating coatings, intermediate layers of nickel can be formed. The stings of modern soldering irons are protected by a layer of nickel, the sting of bare copper slowly dissolves in tin, losing its shape.
W - Tungsten. Refractory metal, melting point 3422 degrees Celsius, which determines its main use - the filament and electrodes.
Filament. In incandescent bulbs, in halogen bulbs, the spiral is made of tungsten, heated by electric current to white heat, while maintaining its shape. Also, the cathodes in radio tubes are made of tungsten, but they do not heat up to such high temperatures as lighting lamps, a special coating on the cathode allows thermionic emission to occur at low temperatures.

Powerful incandescent lamp from the projector. Even the refractory tungsten evaporates over time and settles on the walls of the flask in the form of a dark deposit. This lack of halogen lamps.

The filament of this halogen lamp is made of tungsten. Halogen, usually iodine vapor, chemically binds tungsten evaporating from the filament and returns it to the filament, which makes it possible to raise the filament temperature of the spiral and reduce the lamp envelope without fear that tungsten will gradually settle on the walls of the flask.
Electrodes of arc lamps and welding electrodes. In xenon arc lamps, mercury arc lamps, the electrodes must withstand the temperature of the electric arc, while not melting and not changing its shape, which is only by force of tungsten. Also, electrodes for welding with non-consumable electrodes are made of tungsten (TIG welding).
Anodes of x-ray tubes. The flow of electrons from the cathode in the X-ray tube, which is accelerated by high voltage, is inhibited by bombarding the anode, heating it up very much, so such anodes, especially if they do not have water cooling, are often made of tungsten. However, in physical laboratories, anodes made of copper or cobalt are often used due to the characteristics of the x-ray spectrum from such
anodes.
Tungsten is not a very plastic material, so it is unlikely that it will be possible to straighten and use the spiral of a incandescent lamp according to your understanding. If you suddenly need a tungsten rod - any welding shop will be useful to you, an electrode for a TIG torch without lanthanum and other additives. Wire for the filament homemade equipment is easy to buy on eBay.
Electrode color marking:
Hg - Mercury. At room temperature - brilliant, liquid metal gathering into balls. For environmental reasons, mercury use is declining, but it has been widely used in older devices, and therefore deserves mention.
Like most metals, mercury forms alloys. But mercury, being liquid at room temperature, is able to alloy with metals without additional heating, to dissolve them. A metal dissolved in mercury, an alloy of metal with mercury is called "amalgam".
Liquid contact in position sensors, mercury electrocontact thermometers.
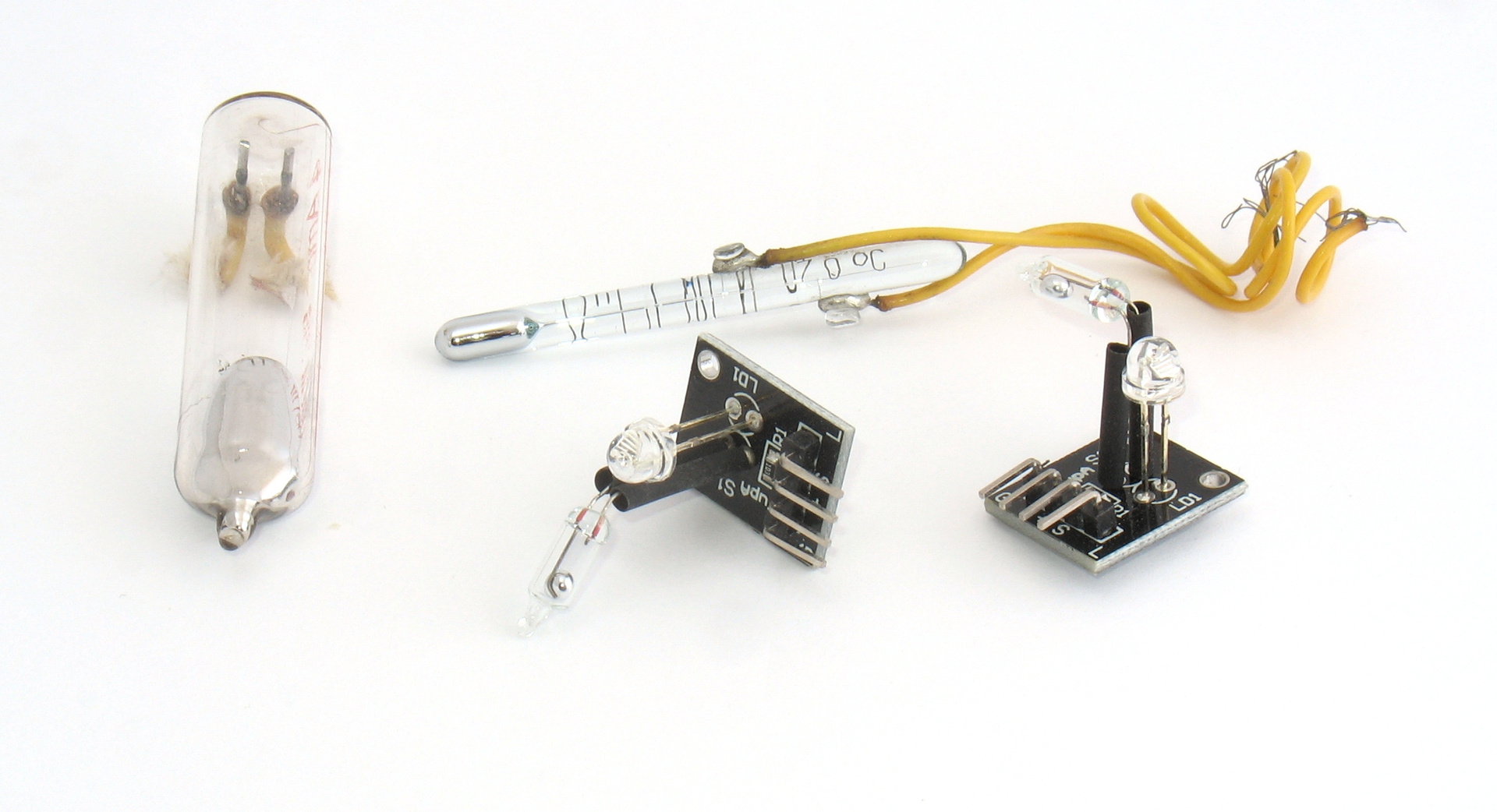
Various mercury devices. On the left - a powerful mercury switch, closing / opening the circuit when tilted. Below on the black shawls - similar Chinese mercury switches - position sensors from the children's set with Arduino. Above - a flask of a mercury electrocontact thermometer. The wires are fused into glass so that at a temperature of 70 ° C a mercury column in the capillary closes the circuit (the temperature is indicated on the body).
In thermometers. Low freezing point, high boiling point and high coefficient of thermal expansion make mercury one of the most convenient substances for laboratory and medical thermometers. In household thermometers, mercury has not been used for a long time.
In manometers and barometers. Mercury is heavy, therefore, 70–80 cm in height of the mercury column is sufficient to balance the atmospheric pressure. Although mercury barometers are largely out of use, pressure units “millimeter of mercury” and in vacuum technology - “micron mercury” and “torr” (rounded version mm Hg) are still used today. Normal atmospheric pressure is 760 mm. Hg Art.
In normal elements. Battery ( Attempting to power a homemade battery from such a battery will result in a failure - the battery has a large internal resistance (on the order of units of kOhm) and is not intended to give currents more than hundredths of a microampere, and even then
breaks. ) with electrodes from liquid mercury, in which mercury and cadmium sulfates are dissolved, has an emf known and stable to units of microvolts (theoretically 1.018636 V at 20 ° C). Such elements are still used in metrology as reference voltage sources, although they are superseded by semiconductor circuits. The vessel with mercury in a normal element is sealed, but it is glass, and there is a lot of mercury in it. Therefore, be careful if you find somewhere a round iron can with a bakelite lid, terminals and the inscription "normal element" on bakelite. Inside it is a glass flask with very dangerous substances.


The element is normal saturated, NE-65, accuracy class 0,005. The appearance of the body of normal elements may vary. Below - the contents of the body, visible mercury in the bottom of the flasks. Such items must be disposed of by a specialized organization.
In diffusion vacuum pumps. A jet of mercury vapor emerging from the nozzle at high speed captures air molecules and pulls them out of the pumped volume. Then mercury vapor is condensed by cooling with liquid nitrogen and used again. Pumps of this type were once used to pump out radio tubes. Now, instead of mercury, non-toxic and liquid nitrogen-free silicone oils are used, but in
Some laboratories still have old mercury systems.
Mercury vapor - working gas fluorescent lamps. Despite the fact that the fluorescent lamp must contain a small amount of mercury, in some lamps mercury is added from the heart, and it is clear that a mercury ball rolls in the flask. When excited by electric current, mercury vapor emits a bright light, mainly in the blue and ultraviolet regions. In addition to them, the spectrum of mercury has bright yellow and green doublets, by the presence of which a mercury lamp can be easily distinguished from any other by looking at it through a prism or reflection in a compact disc. Special mercury lamp in laboratories is used as a source of green light with a known wavelength.
In powerful thyratrons and mercury rectifiers. It is used in the same way as in mercury lamps. Powerful mercury valves were widely used to power locomotives on railways and in other similar tasks before the advent of semiconductor devices.
As a solvent for metals in the separation of gold and platinum from raw materials by amalgamation and in the production of mirrors. Mercury is evaporated, the metal remains. Sometimes this process is incorrectly called "refining", confusing it with a completely different way of isolating precious metals.
In mercury time counters. In the old technique, the mercury capillary coulometer was used as a counter of hours, which the device worked. Brilliant design for simplicity and reliability. Alas, there is no such thing in my collection, but here is a good video .
In amalgam dental fillings. There are to this day, especially in the United States.
All products containing mercury must be disposed of by a specialized service. It is unacceptable to throw them out with household garbage in order to avoid mercury accumulation in the dump.
All mercury spills should be collected and the surfaces should be demercurated. Mercury evaporates well at room temperature, so a mercury ball rolled into the gap for a long time will poison the air.
If your product has broken with mercury, then take the following steps:
1. Open the vents and provide airing.
2. Call the specialized demercurization service in your city. Professionals will not only remove mercury properly, but also measure mercury vapor concentrations.
in room. If suddenly there is no demercurization service in your city, you are far from civilization, then the demercurization process will have to be continued independently.
3. Collect visible mercury balls in an airtight container. It is convenient to put them together using two well-cut sheets of paper, merging the balls into a prepared container. The smallest balls of mercury from the cracks can be pulled out with the help of a spirint, or brushes made of metal that are wetted by mercury (for example, copper). Of course, after using such a "tool" will be contaminated with mercury and must be disposed of.
Then, using chemical agents, the remaining mercury that is not visible to the eye is converted into non-volatile but still poisonous salts, which can be safely removed from the surface with detergents. To do this, use a 0.2% aqueous solution of sodium permanganate ("potassium permanganate") acidified by adding 0.5% hydrochloric acid or a 20% solution of ferric chloride (the one with which the boards are poisoned). Contrary to the indications in the old books, sleeping the spill site with sulfur powder is not effective.
4. Thoroughly rinse the treated area with water and detergent.
5. All collected mercury and contaminated items should be hermetically packed and handed over to a specialized organization.
What is definitely not worth doing when spilling mercury:
1. Panic and hurry. Sometimes, in small accidents, panic and haste do more harm than the accident itself. I remember the bike, recorded Yu.A.Zolotov:
2. Trying to collect the mercury with a vacuum cleaner, the vacuum cleaner only in the turbo mode will shatter and evaporate the mercury balls, as a result, the entire room and the vacuum cleaner itself will be contaminated with rust. Similarly, you should not use brooms, brushes to collect mercury, they only scatter and crush balls of mercury.
3. Drain mercury into the sink or toilet. Mercury is much heavier than water, so it will settle forever in the first bending of the pipe - in a hydraulic seal or knee.
Some children played with mercury balls as a child, and "there was nothing with them." Indeed, contrary to popular belief, metallic mercury is not dangerous at short-term contact. The reason for the low toxicity of metallic mercury is its poor bioavailability. Water insoluble and chemically inert, almost like noble metals, it cannot quickly enter the body.
Inhalation of mercury vapor is dangerous, and this is practically the only way it enters the body. Touching mercury with your fingers adds no additional danger. Moreover, even the ingestion of mercury usually passes without health effects. Mercury is chemically quite inert and leaves the body naturally. Therefore, it is not the cause of acute poisonings, but sluggish chronic ones, manifested in a slow gradual deterioration in health and not always in time diagnosed by doctors. This is exactly what mercury is and insidious: a small ball, rolled under the baseboard, will evaporate and poison the air in the apartment for years, and the residents will not understand what and why they are ill.
Soluble mercury compounds are much more dangerous, and they are formed when mercury in one way or another enters the body of humans, animals or plants. The record for toxicity belongs to dimethyl mercury - it is a terribly toxic substance known to mankind, so toxic that at the first opportunity they look for a less dangerous alternative if they have to work with it. A drop of dimethyl mercury can kill a person through rubber gloves, and the first symptoms of poisoning can appear only the next day.
If you throwing mercury away from home think that the problem has been fixed, then you are seriously mistaken. Mercury - cumulative poison, capable of accumulation in living organisms and transfer further along the food chain. An example of human poisoning with mercury is Minamata disease. Mercury from a discarded fluorescent lamp will poison you, if not you, then your descendants.
If you find mercury anywhere, do not try to sell it. Mercury and its salts are considered potent toxic substances (Art. 234 of the Criminal Code of the Russian Federation). For mercury-containing devices of factory production that meet official standards, the ban does not apply. Found mercury and faulty mercury-containing devices should be handed over for recycling to specialized services in your city. The only widely available source of mercury (if it is suddenly needed in scientific work) is medical thermometers.
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final

Welcome to CAT (TRAFFIC)
Iron
Fe is iron. The main structural material in industry is also used in electrical engineering. Bad, compared to copper, electrical conductivity is compensated by a very low price. And, more importantly in Russia, less attractive for metal hunters, the grounding from a thick rusty pipe will stand without protection longer than a beautiful copper bus.
In the technique of iron is used almost exclusively in the form of alloys with carbon - iron and steel. The properties of steels of different grades are very different: from soft to hard tool.
Application examples
Hardware. Screws, washers, nuts of steel are made in large quantities on specially designed equipment for this. Hardware from other metals are very rare and much more expensive. Therefore, in most cases, the copper tip of the copper wire will be drawn to the copper bus with a steel bolt. Also important is the high strength of steel, the copper bolt does not tighten with the force of steel. Pay attention to the numbers on the bolt head: they indicate its strength. The larger the number, the more you can tighten the bolt.
Terminal blocks, connectors. The well-known "nuts" contain steel plates with a protective coating against corrosion. Also, the use of steel is necessary to prevent galvanic corrosion when connecting copper and aluminum wires.

Connector "nut". Inside the plastic shell, a set of steel plates with screws allows you to make a branch from the cable core without cutting the core itself. Also allows you to go from aluminum to copper.
Ground loops. Electrical safety requirements require grounding. Often, in industrial conditions, the grounding bus is made of rolled steel, fixed along the perimeter of the wall. Poor electrical conductivity of steel is compensated by a large cross section of the conductor. In many cases, safety regulations and standards prescribe that the grounding parts should be made of steel for reasons of mechanical strength.

Steel strip, the envelope of the column - the ground bus.
Magnetic properties of steel are widely used - transformer cores and chokes are assembled from steel plates.
disadvantages
Corrosion. Iron rust, while the density of rust below the density of the original iron, because of this, the design swells . Therefore, iron is coated with protective coatings - galvanized, tinned, chrome-plated, painted, etc. Different steel grades are subject to corrosion to varying degrees, and according to the law of meanness, it is those that are most easily processed on machines that rust the most.
Gold
Au - Gold. The most stupid precious metal. It has the least application in technology of all precious metals, but is a symbol of wealth. Surprisingly more expensive than platinum (2017), which is devoid of common sense and is only the result of speculation.
Application examples
Contact Covers Due to the fact that gold does not oxidize in air, the contacts are covered with a very thin layer of gold.

Gold coating on various electronic components: coating on the contacts of the board for installation in the slot, coating on the contacts of the membrane buttons of the mobile phone, coating on the pins of the processor.
Corrosion protection. In some demanding applications, a gold coating is used to protect the conductors from corrosion (mainly military). Once the gold coating was the only way to protect electronic components from corrosion in the jungle, so many old radio components have even gilded cases. And now they usually just fill the board with a compound in a “brick”.
Interesting facts about gold
- Gold is one of four metals that has a shade in its non-oxidized state. All other metals are white (gold and cesium have a yellowish color, copper is reddish and golden in alloys, osmium has a blue tint).
- The density of gold differs slightly from the density of tungsten (19.32 g / cm 3 for gold, 19.25 g / cm 3 ); this is used to fake gold bars, covering a tungsten ingot with a layer of gold. Perhaps this is one of the reasons why Americans do not allow anyone to verify the authenticity of their gold reserves. And, perhaps, therefore they did not give Germany their gold immediately.
- It is possible to extract gold chemically from a mountain of old electronics, but this is not always economically feasible and is prosecuted by law (Art. 191, 192 of the Criminal Code of the Russian Federation).
Nickel
Ni - Nickel. A wonderful metal, but in electronic technology the main application - as a cheap alternative to gold - is the coating of contacts. If the contact is coated with white shiny metal, then it is most likely nickel.
Application examples
Contact plating. It is applied to copper, plastic, for reliable contact and for decorative purposes. Greedy Chinese sometimes make plastic contacts, covering the top with a layer of nickel and chromium, it looks normal, it even works, but it’s not a question of reliability.

Various nickel-plated connectors for reliable contact.

At the connector on the right, to save metal, the core of the pin is made hollow with plastic filling. Brass nickel-plated tubing from which the pin is made, not the worst option.
Tokovody at the lamps. Platinite alloy (46% Ni, 0.15% C, the rest is Fe) does not contain platinum, but has a linear thermal expansion value very close to platinum, which allows it to be made of reliable electrodes passing through glass. When the temperature changes, such electrodes do not cause glass cracking and loss of tightness.
Intermediate protective layers. To protect against corrosion, mutual diffusion of metals when creating coatings, intermediate layers of nickel can be formed. The stings of modern soldering irons are protected by a layer of nickel, the sting of bare copper slowly dissolves in tin, losing its shape.
Tungsten
W - Tungsten. Refractory metal, melting point 3422 degrees Celsius, which determines its main use - the filament and electrodes.
Application examples
Filament. In incandescent bulbs, in halogen bulbs, the spiral is made of tungsten, heated by electric current to white heat, while maintaining its shape. Also, the cathodes in radio tubes are made of tungsten, but they do not heat up to such high temperatures as lighting lamps, a special coating on the cathode allows thermionic emission to occur at low temperatures.

Powerful incandescent lamp from the projector. Even the refractory tungsten evaporates over time and settles on the walls of the flask in the form of a dark deposit. This lack of halogen lamps.

The filament of this halogen lamp is made of tungsten. Halogen, usually iodine vapor, chemically binds tungsten evaporating from the filament and returns it to the filament, which makes it possible to raise the filament temperature of the spiral and reduce the lamp envelope without fear that tungsten will gradually settle on the walls of the flask.
Electrodes of arc lamps and welding electrodes. In xenon arc lamps, mercury arc lamps, the electrodes must withstand the temperature of the electric arc, while not melting and not changing its shape, which is only by force of tungsten. Also, electrodes for welding with non-consumable electrodes are made of tungsten (TIG welding).
Anodes of x-ray tubes. The flow of electrons from the cathode in the X-ray tube, which is accelerated by high voltage, is inhibited by bombarding the anode, heating it up very much, so such anodes, especially if they do not have water cooling, are often made of tungsten. However, in physical laboratories, anodes made of copper or cobalt are often used due to the characteristics of the x-ray spectrum from such
anodes.
Sources
Tungsten is not a very plastic material, so it is unlikely that it will be possible to straighten and use the spiral of a incandescent lamp according to your understanding. If you suddenly need a tungsten rod - any welding shop will be useful to you, an electrode for a TIG torch without lanthanum and other additives. Wire for the filament homemade equipment is easy to buy on eBay.
Electrode color marking:
- Green is pure tungsten.
- Red, orange - tungsten + thorium (Radioactive! Do not grind, do not cut - dust is dangerous!).
- Blue - tungsten + complex mixture.
- Black, yellow, blue - tungsten + lanthanum.
- Gray - tungsten + cerium.
- White - tungsten + zirconium.
Mercury
Hg - Mercury. At room temperature - brilliant, liquid metal gathering into balls. For environmental reasons, mercury use is declining, but it has been widely used in older devices, and therefore deserves mention.
Like most metals, mercury forms alloys. But mercury, being liquid at room temperature, is able to alloy with metals without additional heating, to dissolve them. A metal dissolved in mercury, an alloy of metal with mercury is called "amalgam".
Application examples
Liquid contact in position sensors, mercury electrocontact thermometers.

Various mercury devices. On the left - a powerful mercury switch, closing / opening the circuit when tilted. Below on the black shawls - similar Chinese mercury switches - position sensors from the children's set with Arduino. Above - a flask of a mercury electrocontact thermometer. The wires are fused into glass so that at a temperature of 70 ° C a mercury column in the capillary closes the circuit (the temperature is indicated on the body).
In thermometers. Low freezing point, high boiling point and high coefficient of thermal expansion make mercury one of the most convenient substances for laboratory and medical thermometers. In household thermometers, mercury has not been used for a long time.
In manometers and barometers. Mercury is heavy, therefore, 70–80 cm in height of the mercury column is sufficient to balance the atmospheric pressure. Although mercury barometers are largely out of use, pressure units “millimeter of mercury” and in vacuum technology - “micron mercury” and “torr” (rounded version mm Hg) are still used today. Normal atmospheric pressure is 760 mm. Hg Art.
In normal elements. Battery ( Attempting to power a homemade battery from such a battery will result in a failure - the battery has a large internal resistance (on the order of units of kOhm) and is not intended to give currents more than hundredths of a microampere, and even then
breaks. ) with electrodes from liquid mercury, in which mercury and cadmium sulfates are dissolved, has an emf known and stable to units of microvolts (theoretically 1.018636 V at 20 ° C). Such elements are still used in metrology as reference voltage sources, although they are superseded by semiconductor circuits. The vessel with mercury in a normal element is sealed, but it is glass, and there is a lot of mercury in it. Therefore, be careful if you find somewhere a round iron can with a bakelite lid, terminals and the inscription "normal element" on bakelite. Inside it is a glass flask with very dangerous substances.


The element is normal saturated, NE-65, accuracy class 0,005. The appearance of the body of normal elements may vary. Below - the contents of the body, visible mercury in the bottom of the flasks. Such items must be disposed of by a specialized organization.
In diffusion vacuum pumps. A jet of mercury vapor emerging from the nozzle at high speed captures air molecules and pulls them out of the pumped volume. Then mercury vapor is condensed by cooling with liquid nitrogen and used again. Pumps of this type were once used to pump out radio tubes. Now, instead of mercury, non-toxic and liquid nitrogen-free silicone oils are used, but in
Some laboratories still have old mercury systems.
Mercury vapor - working gas fluorescent lamps. Despite the fact that the fluorescent lamp must contain a small amount of mercury, in some lamps mercury is added from the heart, and it is clear that a mercury ball rolls in the flask. When excited by electric current, mercury vapor emits a bright light, mainly in the blue and ultraviolet regions. In addition to them, the spectrum of mercury has bright yellow and green doublets, by the presence of which a mercury lamp can be easily distinguished from any other by looking at it through a prism or reflection in a compact disc. Special mercury lamp in laboratories is used as a source of green light with a known wavelength.
In powerful thyratrons and mercury rectifiers. It is used in the same way as in mercury lamps. Powerful mercury valves were widely used to power locomotives on railways and in other similar tasks before the advent of semiconductor devices.
As a solvent for metals in the separation of gold and platinum from raw materials by amalgamation and in the production of mirrors. Mercury is evaporated, the metal remains. Sometimes this process is incorrectly called "refining", confusing it with a completely different way of isolating precious metals.
In mercury time counters. In the old technique, the mercury capillary coulometer was used as a counter of hours, which the device worked. Brilliant design for simplicity and reliability. Alas, there is no such thing in my collection, but here is a good video .
In amalgam dental fillings. There are to this day, especially in the United States.
Toxicity
All products containing mercury must be disposed of by a specialized service. It is unacceptable to throw them out with household garbage in order to avoid mercury accumulation in the dump.
All mercury spills should be collected and the surfaces should be demercurated. Mercury evaporates well at room temperature, so a mercury ball rolled into the gap for a long time will poison the air.
Demercurization
If your product has broken with mercury, then take the following steps:
1. Open the vents and provide airing.
2. Call the specialized demercurization service in your city. Professionals will not only remove mercury properly, but also measure mercury vapor concentrations.
in room. If suddenly there is no demercurization service in your city, you are far from civilization, then the demercurization process will have to be continued independently.
3. Collect visible mercury balls in an airtight container. It is convenient to put them together using two well-cut sheets of paper, merging the balls into a prepared container. The smallest balls of mercury from the cracks can be pulled out with the help of a spirint, or brushes made of metal that are wetted by mercury (for example, copper). Of course, after using such a "tool" will be contaminated with mercury and must be disposed of.
Then, using chemical agents, the remaining mercury that is not visible to the eye is converted into non-volatile but still poisonous salts, which can be safely removed from the surface with detergents. To do this, use a 0.2% aqueous solution of sodium permanganate ("potassium permanganate") acidified by adding 0.5% hydrochloric acid or a 20% solution of ferric chloride (the one with which the boards are poisoned). Contrary to the indications in the old books, sleeping the spill site with sulfur powder is not effective.
4. Thoroughly rinse the treated area with water and detergent.
5. All collected mercury and contaminated items should be hermetically packed and handed over to a specialized organization.
What is definitely not worth doing when spilling mercury:
1. Panic and hurry. Sometimes, in small accidents, panic and haste do more harm than the accident itself. I remember the bike, recorded Yu.A.Zolotov:
Once, when a professor at Moscow State University, Alexei Nikolaevich Kost, was conducting a workshop on organic chemistry, one of the students broke a flask with ether and his vapors broke out. A panic broke out, someone came running with a carbon dioxide fire extinguisher and barely put out the fire. All this time, Kost was completely deadpan at his desk and talking to someone. Then, when everyone calmed down, he approached the scene of the incident and ordered:
- Matches!
He was given a box, he struck a match and threw it into the still dry airy puddle. The fire flashed again, everyone was taken aback. And Kost, without fussing, took a fire blanket, deftly covered them with a flame and spoke:
- It is necessary to burn skillfully!
2. Trying to collect the mercury with a vacuum cleaner, the vacuum cleaner only in the turbo mode will shatter and evaporate the mercury balls, as a result, the entire room and the vacuum cleaner itself will be contaminated with rust. Similarly, you should not use brooms, brushes to collect mercury, they only scatter and crush balls of mercury.
3. Drain mercury into the sink or toilet. Mercury is much heavier than water, so it will settle forever in the first bending of the pipe - in a hydraulic seal or knee.
A few words about mercury toxicology
Some children played with mercury balls as a child, and "there was nothing with them." Indeed, contrary to popular belief, metallic mercury is not dangerous at short-term contact. The reason for the low toxicity of metallic mercury is its poor bioavailability. Water insoluble and chemically inert, almost like noble metals, it cannot quickly enter the body.
Inhalation of mercury vapor is dangerous, and this is practically the only way it enters the body. Touching mercury with your fingers adds no additional danger. Moreover, even the ingestion of mercury usually passes without health effects. Mercury is chemically quite inert and leaves the body naturally. Therefore, it is not the cause of acute poisonings, but sluggish chronic ones, manifested in a slow gradual deterioration in health and not always in time diagnosed by doctors. This is exactly what mercury is and insidious: a small ball, rolled under the baseboard, will evaporate and poison the air in the apartment for years, and the residents will not understand what and why they are ill.
Soluble mercury compounds are much more dangerous, and they are formed when mercury in one way or another enters the body of humans, animals or plants. The record for toxicity belongs to dimethyl mercury - it is a terribly toxic substance known to mankind, so toxic that at the first opportunity they look for a less dangerous alternative if they have to work with it. A drop of dimethyl mercury can kill a person through rubber gloves, and the first symptoms of poisoning can appear only the next day.
If you throwing mercury away from home think that the problem has been fixed, then you are seriously mistaken. Mercury - cumulative poison, capable of accumulation in living organisms and transfer further along the food chain. An example of human poisoning with mercury is Minamata disease. Mercury from a discarded fluorescent lamp will poison you, if not you, then your descendants.
additional information
If you find mercury anywhere, do not try to sell it. Mercury and its salts are considered potent toxic substances (Art. 234 of the Criminal Code of the Russian Federation). For mercury-containing devices of factory production that meet official standards, the ban does not apply. Found mercury and faulty mercury-containing devices should be handed over for recycling to specialized services in your city. The only widely available source of mercury (if it is suddenly needed in scientific work) is medical thermometers.
Links to parts of the manual:
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final
All Articles