Are zombie cell cells a false sign in the fight against aging?
Correlation does not mean causality. These phrases are hammered into the head of all students of statistics from the first classes. At the same time, the correlation, of course, hints at this very causality — often with two correlating parameters, if not a direct causal link, then at least a common external cause. One of my favorite examples: ice cream sales correlate well with the number of drowned people, but at the same time both variables do not depend on each other, but are due to the third factor - the weather.
In the fight against aging, new and new hypotheses of some Great Therapy are constantly appearing and quickly coming into fashion, which will help to win a decisive victory in this fight. Not so long ago, it was Her Majesty Telomerase, but a couple of years ago she was removed from the throne of the senolithic - a means of dealing with senescent cells . These are zombie cells that not only do not fulfill their functions and refuse to die, but also poison everything around them, emitting a cocktail of pro-inflammatory substances called “ senescence-associated secretory phenotype ” or SASP.

True, as has often happened, the correlation may again try to show its cunning and direct us on the wrong track: the fact that the body becomes more senescent cells as it ages does not mean that they are its driver. And it seems that they may turn out to be such a false trail. The latest research by Andrei Gudkov - professor, doctor of biological sciences, founder and scientific director of the biotechnology company Cleveland BioLabs, and generally one of the most successful Russian biologists abroad - the results for which he presented in January 2017 at the Scripps Conference on Aging Biology, make me more and more make sure of it. In general, for me it is very interesting, I would even say revolutionary, data. Here is his full video presentation, look, you will not regret:
What did Andrew say of such a revolutionary? Here's what:




What is Andrei Gudkov's hypothesis about this? What, in his opinion, explains all these mysterious observations summarized on the slide below?

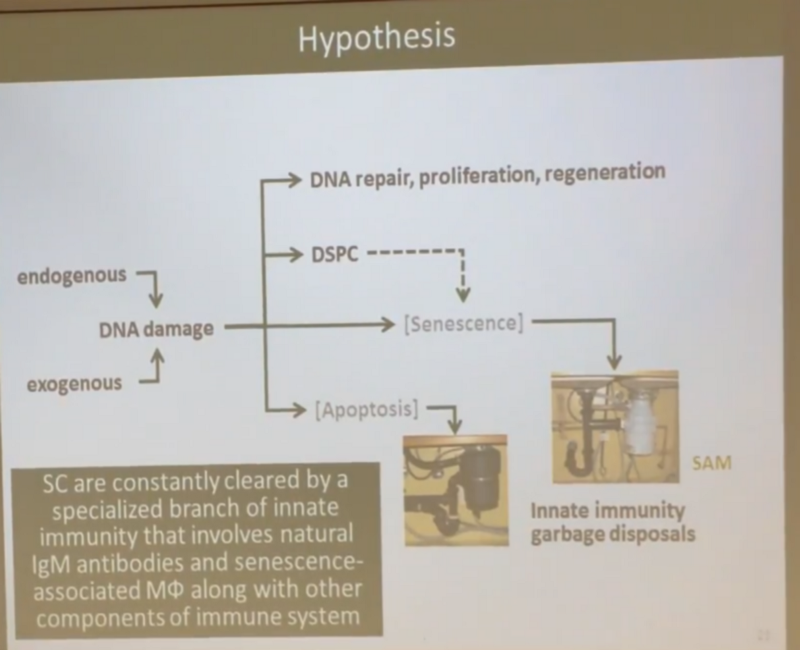
The hypothesis is as follows: as DNA damage occurs, most cells (not killed immediately by apoptosis), in which these injuries could not be repaired, are not immediately transformed into senescent cells (as in the incorrect, from the slide above, according to Andrew’s opinion today) ), and freezes in a certain condition, named by him DSPC ( Dormant Senescent-Prone Cells , or “hidden pro-senescent cells”).
That is, these cells continue to live and function, and become sensate only if they have a need to divide, but then the (innate) immune system comes into play, in which the function of catching and killing senescent cells is well established - macrophages and immunoglobulins are responsible for this M (IgM). Schematically, Andrei’s new hypothesis looks like this:
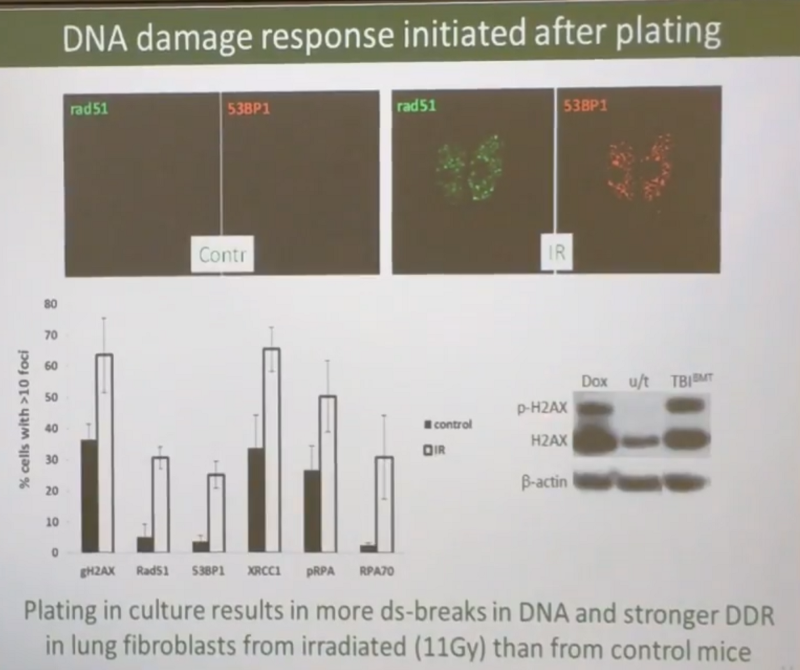
True, Andrew believes that all of the above is true only for mesenchymal cells, and after irradiation, epithelial cells follow the highest path on the slide above, that is, DNA breakages are repaired in them immediately. In confirmation of the fact that the mesenchymal cells are full of breakdowns, Andrew cites data that irradiated mice have an order of magnitude more DNA strand breaks:

Did Andrew test epithelial cells for double-stranded breaks in order to test his hypothesis that the damage in them is immediately repaired, I do not know. But on the basis of experimental data on mesenchymal cells, Andrew believes that after irradiation, almost 100% of them become so “pro-sensative” (DSPC), as he writes on this slide:
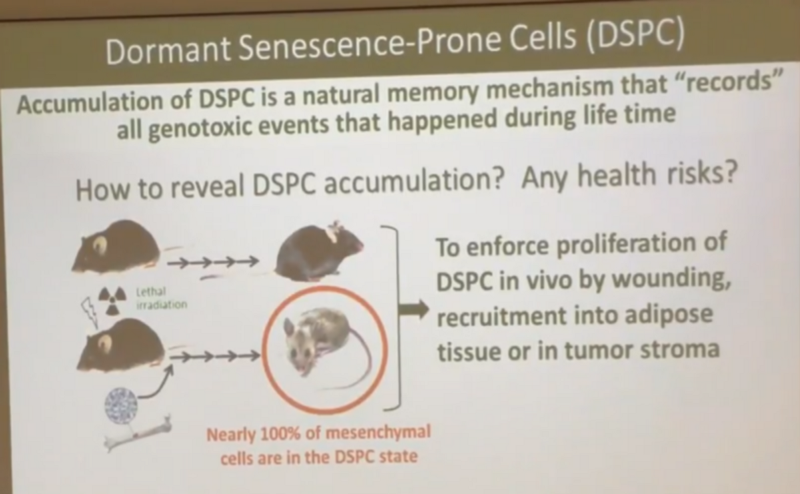
And it is precisely the fact that when they divide, they become senescent and are killed by the immune system, which explains, in Andrew’s opinion, the best resistance of irradiated mice to cancer - the tumor cannot grow quickly, since new blood vessels designed to supply it with blood grow in such The mice are much slower, since the precursor cells of these vessels are all pro-enesencent.
Andrew proved experimentally that the cell culture of the cell culture almost immediately turns into senescent cells and does not grow, unlike the cells of control mice:

What else, according to Andrew, confirms this hypothesis, is that on a high-calorie diet (or highly fatty , to be precise), irradiated mice die even faster. However, in other experiments it was shown that a fat diet also shortens the life of mice by itself, and the fact that the non-irradiated control group on the fat diet also lost about 10% of the population simultaneously with the irradiated group, in my eyes, gives rise to some doubts . Therefore, I would very much like to see the full survival curve for the unirradiated control on a fat diet:

How did Andrew show that it is the immune system that is responsible for controlling and eliminating senescent cells? Very beautiful. He placed the senescent cells in a kind of construction resembling a metal cage for shark divers, and implanted these designs into a mouse. And then I looked at what sharks swim to these “cages”. Sharks turned out to be mainly macrophages (with the usual entourage of other immune cells — eosinophils, etc.).
Here on this graph it is shown that without protective “cells” senescent cells disappear very quickly (population decreases 100 times) after implantation in a mouse (green curve), and when they are placed in a protective capsule, making them inaccessible to any other cells, their numbers practically does not decrease (blue curve):

And here are the sharks: macrophages. And, surprisingly, these macrophages themselves begin to express the senescent marker of beta-galactosidase, which was previously considered to be a marker of exclusively senescent cells. Why this is happening, I have not yet understood, and Andrei, in my opinion, too.

Moreover, Andrei showed in another experiment that a significant part of those cells that we previously considered to be senescent are macrophages, which themselves are unlikely to be senescent (that is, they do not secrete SASP — the already mentioned pro-inflammatory cocktail). scattered among a population of true senescent cells, like fighters on the battlefield:
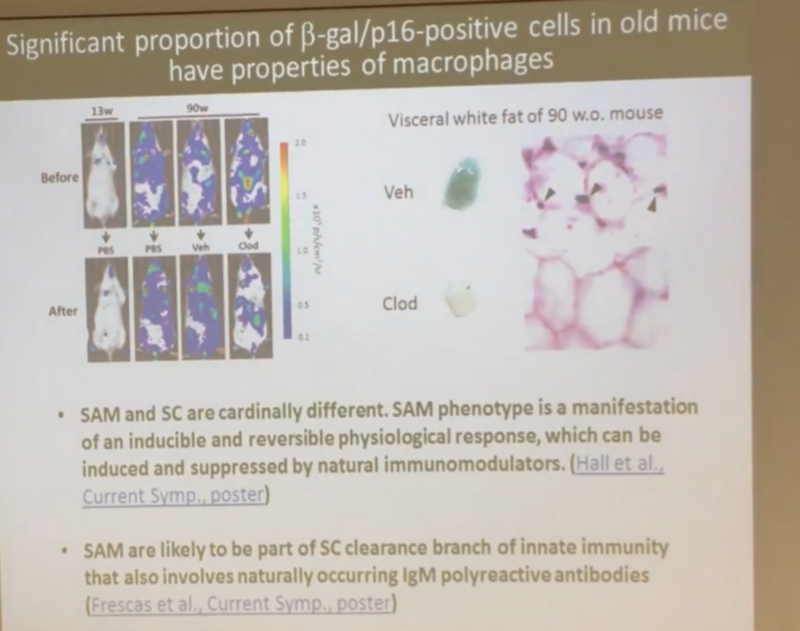
The main question for me and Andrei is why these fighters cope with the senescent cells so well until old age, and then they cope abruptly. Here our points of view diverge. Andrew believes that with age, some resource of the immune system is exhausted, and that is why it ceases to cope with them. And in irradiated mice, this resource is exhausted more quickly, because there are much more senescent cells:
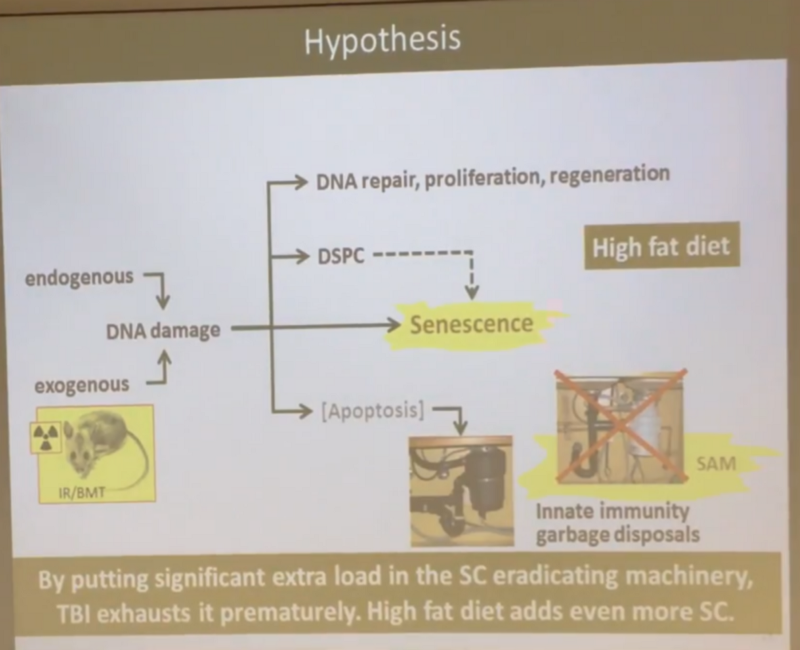
Of course, I disagree with the resource hypothesis. I can not imagine that 11 Gy of radiation, which turns 100% of mesenchymal cells into progenaceous, begins to exhaust the resource only after a year, and then reducing the average life span by only 28%, and the maximum life span by 18–20%.
At the same time, it is very interesting to me why, according to Andrew’s hypothesis, epithelial cells are spared from this fate. It seems to me important to understand what exactly happened in irradiated mice with fast-dividing tissues and how it fits with the hypothesis of pro-senescent cells. After all, there are quite a lot of rapidly renewing populations in the body: intestines, stomach, lungs, reproductive system (the blood system does not count, since it is formed mainly by the bone marrow, which was transplanted to irradiated mice from intact donors):
Also, it is not very clear to me how the mesenchymal cells of irradiated mice managed to continue functioning and correctly synthesizing the right proteins for their vital activity, if they had many times more DNA breakdowns (after all, proteins are built on DNA). By the way, this is another excellent counter-argument against the hypothesis of “aging from the accumulation of mutations” (after all, there is someone else who believes in this hypothesis ...). If you remember, double-stranded DNA breaks in irradiated mice were an order of magnitude larger.
In any case, the concept of a programmed reduction in the quality of reparation mechanisms seems to be much more plausible to me, which in my youth, even after mega-dose of radiation, do not allow the appearance of senescent cells, and in old age, even in control mice, they cause similar numbers.
In this case, a fat diet is a signal for the internal clock to accelerate aging. And calorie restriction is a reverse signal, significantly extending the life of ordinary mice. Yes, and sublethal doses of radiation (25–50 times higher than background), by the way, also prolonged the life of mice by 20%. That, in my understanding, does not fit well with any concepts of resources. By the way, it would be interesting to see the effect of calorie restriction on irradiated mice.
Andrei has his own senolithic , EBS3899 (from Everon Biosciences ), which worked perfectly in cell cultures, but when broadcasting to a living organism was, according to Andrew, much less effective: the effect of increasing ALE by 13% was observed only in male mice, and only if the senolithic was used at the 89th week of life (earlier application did not lead to an increase in the pancreas, as well as its use in females):

Therefore, the main conclusion of Andrew, as I heard him, is that we need to look for tools to influence other aging mechanisms (adjustable keys on the slide), if we want to achieve a much larger increase in lifespan:

And it’s hard to disagree with Andrei.
By the way, perhaps even Ned David, the head of Unity Biotechnology, the largest startup in the development of senoliths in which Peter Thiel and Jeff Bezos invested, agree with him. David has already met twice with Juan Carlos Ispisua Belmonte, the author of my favorite work , and in March 2017 they already discussed some possible next steps.
Well, with great interest we will follow further developments.

In the fight against aging, new and new hypotheses of some Great Therapy are constantly appearing and quickly coming into fashion, which will help to win a decisive victory in this fight. Not so long ago, it was Her Majesty Telomerase, but a couple of years ago she was removed from the throne of the senolithic - a means of dealing with senescent cells . These are zombie cells that not only do not fulfill their functions and refuse to die, but also poison everything around them, emitting a cocktail of pro-inflammatory substances called “ senescence-associated secretory phenotype ” or SASP.

True, as has often happened, the correlation may again try to show its cunning and direct us on the wrong track: the fact that the body becomes more senescent cells as it ages does not mean that they are its driver. And it seems that they may turn out to be such a false trail. The latest research by Andrei Gudkov - professor, doctor of biological sciences, founder and scientific director of the biotechnology company Cleveland BioLabs, and generally one of the most successful Russian biologists abroad - the results for which he presented in January 2017 at the Scripps Conference on Aging Biology, make me more and more make sure of it. In general, for me it is very interesting, I would even say revolutionary, data. Here is his full video presentation, look, you will not regret:
Experimental Observations
What did Andrew say of such a revolutionary? Here's what:
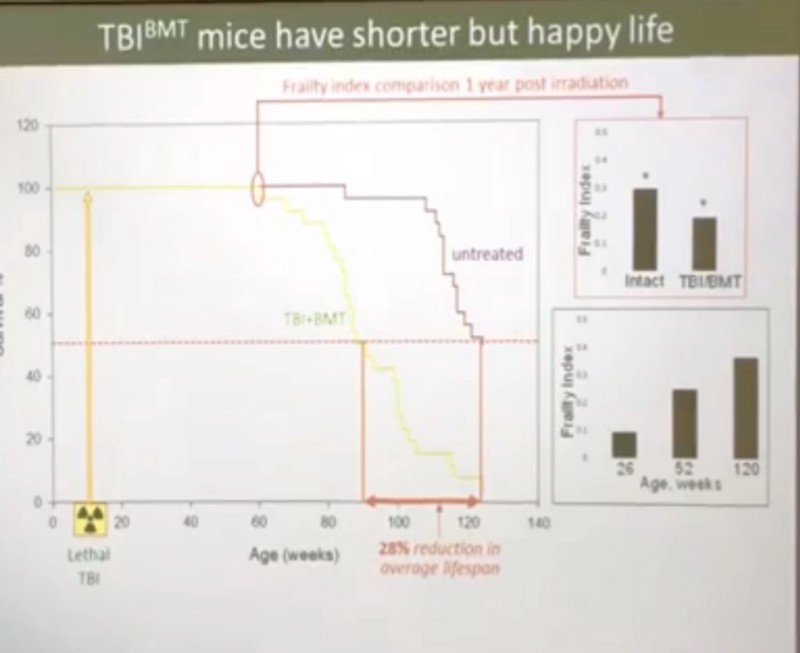
- In mice irradiated at 10 weeks of life with a “lethal” radiation dose of 11 Gy (apparently, this dose is fatal, if donor bone marrow is not transplanted to the mice immediately after irradiation, which was done in Gudkov’s experiments), no sensate cells are detected. 30 weeks after irradiation. Which is very strange, because in vitro (in cell cultures) irradiation is a guaranteed way to send a significant part of the cells to a senescent state (here, the dark clusters in the photo below are senescent cells; pay attention to how many there are when the cell culture is irradiated with a dose of 11 ):

- Moreover, the brittleness index in irradiated mice a year after radiation was better than in control mice that did not receive irradiation, and the irradiated mice lived not dramatically less than the control mice (mean RV at 90 weeks versus 120 control, and the maximum RV of irradiated mice was 120 weeks vs. 135 weeks in another experiment):

- At the same time, not only senescent cells are not detected in mice, but the cytokine profile of the inflammatory response does not differ from control mice, which cannot be said of unirradiated old mice, in which the inflammatory response is much higher (the same inflammaging ). That is, the immune system after irradiation works perfectly at the same level as that of unirradiated peers. Here, however, it is important to remember that the bone marrow in donated mice is donor, and that it is the source of hematopoietic stem cells, the precursors of the vast majority of immune cells (tissue macrophages and other tissue immune residents are an exception). But in any case, it is very strange to see that irradiation does not affect the inflammatory response, or the number of senescent cells. The slide below shows 40-week irradiated (3rd row) mice, compared to 40-week (1st row) and 98-week (2nd row) non-irradiated mice. It is clearly seen that senescent cells (blue spots) are present only in non-irradiated 98-week-old mice. The last column on the right is the cytokine profile of the immune response:

- By the way, the transcript of the irradiated and non-irradiated 40-week-old mice is almost identical, in contrast to the transcriptome of 98-week-old mice:

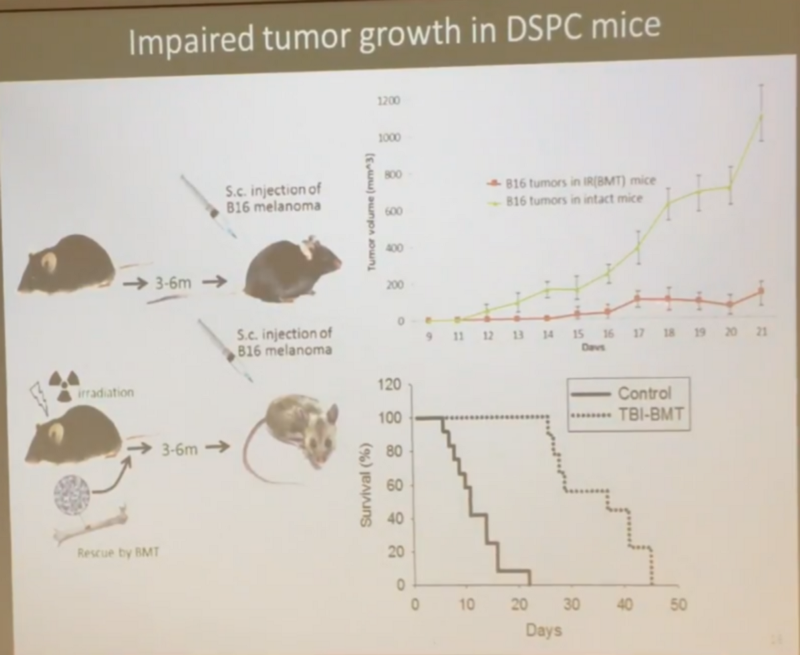
- And cancer resistance in irradiated mice is generally higher. In a model of induced cancer, when mice were injected with melanoma cells ( B16 melanoma cells ), irradiated mice lived longer than unirradiated, even though they had much more lung metastases:

- And cancer tumors in irradiated mice grew much slower (red curve versus green), and the average survival was 3.5 times higher than unirradiated (that is, even better than the equal average survival in the previous experiment):

The hypothesis of Andrei Gudkov
What is Andrei Gudkov's hypothesis about this? What, in his opinion, explains all these mysterious observations summarized on the slide below?

The hypothesis is as follows: as DNA damage occurs, most cells (not killed immediately by apoptosis), in which these injuries could not be repaired, are not immediately transformed into senescent cells (as in the incorrect, from the slide above, according to Andrew’s opinion today) ), and freezes in a certain condition, named by him DSPC ( Dormant Senescent-Prone Cells , or “hidden pro-senescent cells”).
That is, these cells continue to live and function, and become sensate only if they have a need to divide, but then the (innate) immune system comes into play, in which the function of catching and killing senescent cells is well established - macrophages and immunoglobulins are responsible for this M (IgM). Schematically, Andrei’s new hypothesis looks like this:

True, Andrew believes that all of the above is true only for mesenchymal cells, and after irradiation, epithelial cells follow the highest path on the slide above, that is, DNA breakages are repaired in them immediately. In confirmation of the fact that the mesenchymal cells are full of breakdowns, Andrew cites data that irradiated mice have an order of magnitude more DNA strand breaks:


Did Andrew test epithelial cells for double-stranded breaks in order to test his hypothesis that the damage in them is immediately repaired, I do not know. But on the basis of experimental data on mesenchymal cells, Andrew believes that after irradiation, almost 100% of them become so “pro-sensative” (DSPC), as he writes on this slide:

And it is precisely the fact that when they divide, they become senescent and are killed by the immune system, which explains, in Andrew’s opinion, the best resistance of irradiated mice to cancer - the tumor cannot grow quickly, since new blood vessels designed to supply it with blood grow in such The mice are much slower, since the precursor cells of these vessels are all pro-enesencent.
Andrew proved experimentally that the cell culture of the cell culture almost immediately turns into senescent cells and does not grow, unlike the cells of control mice:

What else, according to Andrew, confirms this hypothesis, is that on a high-calorie diet (or highly fatty , to be precise), irradiated mice die even faster. However, in other experiments it was shown that a fat diet also shortens the life of mice by itself, and the fact that the non-irradiated control group on the fat diet also lost about 10% of the population simultaneously with the irradiated group, in my eyes, gives rise to some doubts . Therefore, I would very much like to see the full survival curve for the unirradiated control on a fat diet:

Immune system - forest orderly
How did Andrew show that it is the immune system that is responsible for controlling and eliminating senescent cells? Very beautiful. He placed the senescent cells in a kind of construction resembling a metal cage for shark divers, and implanted these designs into a mouse. And then I looked at what sharks swim to these “cages”. Sharks turned out to be mainly macrophages (with the usual entourage of other immune cells — eosinophils, etc.).
Here on this graph it is shown that without protective “cells” senescent cells disappear very quickly (population decreases 100 times) after implantation in a mouse (green curve), and when they are placed in a protective capsule, making them inaccessible to any other cells, their numbers practically does not decrease (blue curve):

And here are the sharks: macrophages. And, surprisingly, these macrophages themselves begin to express the senescent marker of beta-galactosidase, which was previously considered to be a marker of exclusively senescent cells. Why this is happening, I have not yet understood, and Andrei, in my opinion, too.

Moreover, Andrei showed in another experiment that a significant part of those cells that we previously considered to be senescent are macrophages, which themselves are unlikely to be senescent (that is, they do not secrete SASP — the already mentioned pro-inflammatory cocktail). scattered among a population of true senescent cells, like fighters on the battlefield:

The main question for me and Andrei is why these fighters cope with the senescent cells so well until old age, and then they cope abruptly. Here our points of view diverge. Andrew believes that with age, some resource of the immune system is exhausted, and that is why it ceases to cope with them. And in irradiated mice, this resource is exhausted more quickly, because there are much more senescent cells:

Of course, I disagree with the resource hypothesis. I can not imagine that 11 Gy of radiation, which turns 100% of mesenchymal cells into progenaceous, begins to exhaust the resource only after a year, and then reducing the average life span by only 28%, and the maximum life span by 18–20%.
At the same time, it is very interesting to me why, according to Andrew’s hypothesis, epithelial cells are spared from this fate. It seems to me important to understand what exactly happened in irradiated mice with fast-dividing tissues and how it fits with the hypothesis of pro-senescent cells. After all, there are quite a lot of rapidly renewing populations in the body: intestines, stomach, lungs, reproductive system (the blood system does not count, since it is formed mainly by the bone marrow, which was transplanted to irradiated mice from intact donors):

Also, it is not very clear to me how the mesenchymal cells of irradiated mice managed to continue functioning and correctly synthesizing the right proteins for their vital activity, if they had many times more DNA breakdowns (after all, proteins are built on DNA). By the way, this is another excellent counter-argument against the hypothesis of “aging from the accumulation of mutations” (after all, there is someone else who believes in this hypothesis ...). If you remember, double-stranded DNA breaks in irradiated mice were an order of magnitude larger.
In any case, the concept of a programmed reduction in the quality of reparation mechanisms seems to be much more plausible to me, which in my youth, even after mega-dose of radiation, do not allow the appearance of senescent cells, and in old age, even in control mice, they cause similar numbers.
In this case, a fat diet is a signal for the internal clock to accelerate aging. And calorie restriction is a reverse signal, significantly extending the life of ordinary mice. Yes, and sublethal doses of radiation (25–50 times higher than background), by the way, also prolonged the life of mice by 20%. That, in my understanding, does not fit well with any concepts of resources. By the way, it would be interesting to see the effect of calorie restriction on irradiated mice.
So what about the senolithic?
Andrei has his own senolithic , EBS3899 (from Everon Biosciences ), which worked perfectly in cell cultures, but when broadcasting to a living organism was, according to Andrew, much less effective: the effect of increasing ALE by 13% was observed only in male mice, and only if the senolithic was used at the 89th week of life (earlier application did not lead to an increase in the pancreas, as well as its use in females):

Therefore, the main conclusion of Andrew, as I heard him, is that we need to look for tools to influence other aging mechanisms (adjustable keys on the slide), if we want to achieve a much larger increase in lifespan:

And it’s hard to disagree with Andrei.
By the way, perhaps even Ned David, the head of Unity Biotechnology, the largest startup in the development of senoliths in which Peter Thiel and Jeff Bezos invested, agree with him. David has already met twice with Juan Carlos Ispisua Belmonte, the author of my favorite work , and in March 2017 they already discussed some possible next steps.
Well, with great interest we will follow further developments.

All Articles