Storm and onslaught of gene therapy of aging

Gene Expression and Gene Therapy
The concept of gene therapy has existed for the past twenty to thirty years. It lies in the fact that the most radical way of dealing with diseases is the destruction of the genetic cause of the disease itself, and not its consequences.
The reason may be a violation of a specific gene (mutation), which is transmitted from parents with hereditary diseases, or a gene mutation already in an adult organism, causing, for example, cancer. In addition, the cause may simply be too high (or, conversely, low) activity of a normal gene, which increases the risk of a chronic disease, such as diabetes or atherosclerosis.
The way to combat these changes is to introduce a new genetic information into the cell, designed to correct the one with which the disease is associated.
First, a little deeper into the theory. The DNA molecule - deoxyribonucleic acid - is the carrier of the code that controls the development and functioning of all living things. DNA contains a program that enables the transformation of a single source cell into a coherently functioning organism consisting of numerous cells, combined into various tissues and organs.

Deciphering the structure of DNA in 1953 was one of the turning points in the history of biology. For this discovery, scientists James Watson, Francis Crick and Maurice Wilkins were awarded the Nobel Prize in Physiology and Medicine in 1962.
DNA is a huge molecule that consists of thousands of small molecules — nucleotides of four different types: guanine (G), cytosine (C), thymine (T), and adenine (A). Nucleotides are connected to each other, forming a chain; DNA molecule consists of 2 such chains, twisted into a double helix.

When connecting 2 chains, the following rules are followed: adenine is always connected with thymine, and guanine - with cytosine. Thus, opposite to a thymine from one thread there will always be adenine from another.
This arrangement allowed us to explain the mechanisms of DNA copying during cell division. The two threads of the helix diverge, and then an exact copy of her ex-partner in a spiral is added to each of them. By the same principle, as with the negative in the photo print positive.
The next revolution in the study of DNA occurred 50 years later, in 2003, when the huge project “The Human Genome” was completed. He allowed to decipher all 19,000 genes of our body and opened up unprecedented opportunities for medicine.

A gene is a portion of a DNA molecule in which a polypeptide (part of a protein molecule) is encoded or functional RNA. Long, he, as a rule, several hundred nucleotides, but there are exceptions. The smallest genes of the human genome - the genes of transport RNA - are only 76 base pairs, and the largest gene, the dystrophin protein, is 2.4 million.
In the most developed organisms, including humans, the genes are often separated by fragments of "meaningless" non-coding DNA. Human DNA is wrapped around the molecular backbone of proteins, with which it forms the chromosome. All human DNA is located in 46 chromosomes.
If we compare the cell with the plant, the DNA will be something like information from the hard disk stored in the office of the plant. In order for the plant to start working, this information must be transmitted to all devices in the workshops of the plant — ribonucleic acids — RNA — perform this role in the cell. And finally, the products that are beginning to be assembled in the shops of the plant under this program are cellular proteins.
The process of reading information from DNA is called "gene expression."

If DNA is coded information about all processes of the body, then proteins are the main executors and controllers of these processes. There are a huge number of different classes of proteins that are involved in all processes important to the body.
There are proteins that shorten the course of chemical reactions in the body; perform the building function - as a kind of reinforcement give shape to the cells and their parts; protect the body by disabling toxins, pathogenic bacteria and viruses; regulate the reading of information from DNA and the synthesis of the corresponding proteins.
There are also proteins that transmit signals between cells, tissues and organs, transport various molecules through cells and different systems; proteins accumulate energy; proteins are receptors - they trigger a cascade of cellular events in response to certain signals from the external environment or from the internal systems of the body; they can perform a motor function - provide the body's movements, for example, muscle contraction.

Gene therapy is an intervention in the work of the cellular "plant" for the production of proteins. It allows both to activate the work of the desired genes and to “turn off” the harmful ones. In the first case, the gene is delivered into the cell, from which the protein necessary for the therapy of the disease begins to be read. And in the second, regulatory RNAs are introduced into the cell, which block the expression of the “harmful” gene.
Most often, blocking of genes is achieved due to the fact that small interfering RNAs (miRNAs) are delivered into the cell, which bind to the RNA of the gene that needs to be turned off. Binding of siRNA and RNA blocks protein synthesis and ultimately leads to RNA degradation.
This process is called RNA interference. It was opened in 1998 by American scientists Andrew Fire and Craig Mello and was recognized as so important that as early as 2006, the Nobel Prize in Physiology and Medicine was awarded for its discovery.
Gene Therapy: Progress and Trends
Gene therapy originated over 25 years ago. The first successful clinical trial (in humans) was conducted in 1989 on the gene therapy of severe combined immunodeficiency. At the moment, gene therapy is booming.
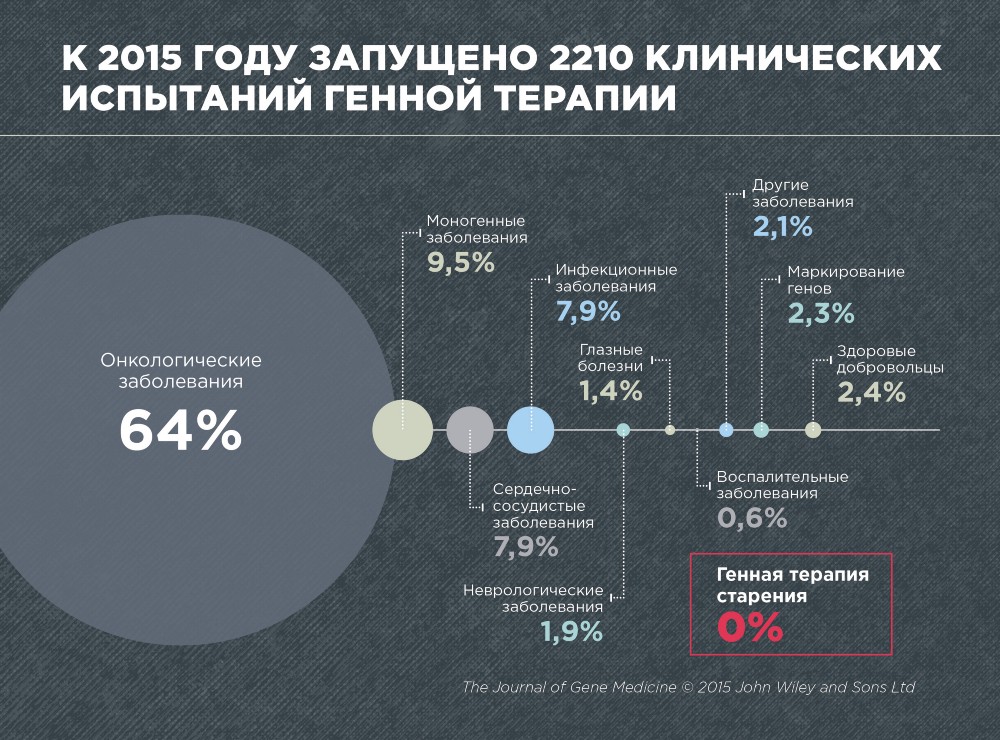
According to the journal Gene Medicine, in 2015, 2210 clinical trials on gene therapy of various diseases were conducted. These are predominantly cancer (64%), monogenic diseases caused by mutations in a single gene (9.5%), cardiovascular (7.9%) and infectious (7.9%).
No single clinical trial has been conducted on gene therapy of aging, which is not surprising, since aging has not yet been recognized as a disease. In addition, gene therapy of aging is still very young and developing area.
For a number of diseases, gene therapy has proven to be quite successful. Among them, hereditary diseases of the immune system - severe combined immunodeficiency, Wiskott-Aldrich syndrome and chronic granulomatous disease; hereditary disease associated with metabolic disorders - adrenoleukodystrophy; hereditary disease of the retina - Leber amaurosis and some forms of cancer.
To date, already 4 gene therapy drugs are approved for sale. In China, Gendicine, a p53-based drug for the treatment of squamous cell head and neck cancer, was released in 2003, and Oncorine (Oncorine), a virus for treating nasopharyngeal carcinoma, was released in 2006. In Europe, in 2012, the company launched production of the drug Glybera (Glybera), intended for the treatment of hereditary lipoprotein lipase deficiency (LPL) by delivering a gene of the same name. And in Russia, the drug Neovasculgen for the treatment of peripheral artery diseases has been approved for sale. It is a gene of VEGF (vascular endothelial growth factor).
The main problem of gene therapy is how to deliver a therapeutic gene (or RNA) to target cells. Usually for this purpose, vectors are used for delivery - carriers of genetic constructs. They do not allow DNA to break down in the blood, provide DNA from the capillaries (small blood vessels) in the tissue and penetration into the cells and into the cell nucleus.
Most often, viruses are used as vectors, since they have very efficient - honed evolution - mechanisms of entry into animal cells. By infecting a cell in nature, the virus delivers its genetic material into the nucleus of this cell and begins to reproduce and accumulate its proteins using the mechanisms of gene expression of the host cell.
Scientists have simplified viruses by removing the genes involved in the pathogenesis and causing the immune response of the body, and turned into vectors for the delivery of genetic material.
 The most popular viruses used in gene therapy are adenoviruses (they are used in 22.2% of clinical studies), as well as retroviruses (they account for 18.4% of works). Only newer vectors are gaining popularity - adeno-associated viruses (6% of clinical trials) and lentiviruses (5% of studies).
The most popular viruses used in gene therapy are adenoviruses (they are used in 22.2% of clinical studies), as well as retroviruses (they account for 18.4% of works). Only newer vectors are gaining popularity - adeno-associated viruses (6% of clinical trials) and lentiviruses (5% of studies).
Adeno-associated (AAV) and lentiviral vectors appear to be the most promising for gene therapy. The first will allow to deliver the genetic structure into the body systemically (that is, into a number of tissues and organs) without side effects. True, they do not embed genetic material into the genome, so that the delivered gene can be lost over time.
If it is necessary to ensure high efficiency of delivery and integration of the delivered construct into the genome, lentiviral vectors should be used. However, they are not suitable for systemic delivery and are used for local administration in a small area of tissue or in cells in a test tube. In addition, they can cause side effects due to embedding in unwanted places of the genome (for example, in proto-oncogenes and cause cancer).

The use of the CRISPR / Cas9 genome editing technology opens up new possibilities in gene therapy. CRISPR / Cas9 allows you to very accurately and safely change the DNA of cells. And if you combine the technology of CRISPR / Cas9 with the delivery with the help of adeno-associated viruses, then this seems to allow a systemic effect on the body and it is absolutely safe to change the genome of a very large number of cells. That is, its use allows combining the advantages of both adeno-associated and lentiviral vectors.
In nature, this system is present in bacteria and archaea. It is used to protect against bacteriophages (bacteria viruses) or other foreign genetic elements. If a cell becomes infected, the CRISPR / Cas system recognizes the sequences of foreign DNA and cuts it. The CRISPR / Cas system was first discovered in 1987, but its functions began to be actively studied only starting from 2005.
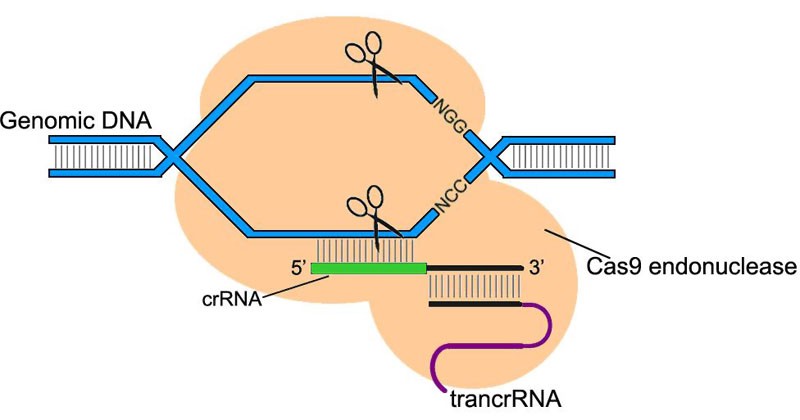
Because CRISPR / Cas super-recognizes and cuts DNA, the scientists decided to adapt it for editing the mammalian genome. Using CRISPR / Cas has exceeded all expectations. It allowed, with a minimum number of errors, both to “turn off” the necessary genes and to integrate new genes into strictly specific regions of the genome.
The CRISPR / Cas9 system consists of the Cas9 endonuclease, an enzyme that cuts DNA, and a guide RNA, which binds only to a well-defined genome sequence. This system allows you to find and cut sections of the genome in the right place, aiming at them directing RNA.
The guide RNA is selected by the researchers themselves, so that the CRISPR / Cas9 system can be aimed at any desired part of the genome.

And most recently, in December 2015, the scientific group Feng Janga modified this system so that it became completely infallible, which was published in the leading scientific journal Science. Scientists have replaced 3 amino acids (the “brick” of which the protein is made) in the endonuclease Cas9, after which the number of errors of such a system has been reduced to almost zero.
The use of CRISP / Cas9 is especially important for gene therapy of aging, where it is necessary to influence the paths of longevity, common to most cells of the body.
Types of delivery vectors and their properties
Adenoviruses (AV) and adeno-associated viruses (AAV) can be used both for highly targeted delivery of genetic material to certain tissues and for systemic delivery to the body (that is, to a large number of organs and tissues). They deliver genetic material very efficiently, penetrating into the nucleus of both dividing and non-dividing cells.
An important feature of these viruses is that they do not integrate the delivered genes into the genome. However, in the case of adeno-associated vectors, it was nevertheless shown that <1% of cases there is an integration (integration) into a certain region of the host genome.
The absence of random gene integration into the genome is a big plus in terms of the safety of these vectors. In this case, there is no risk that the gene will accidentally integrate into the sequence of the proto-oncogene and cause cancer. On the other hand, if a gene is not inserted into the genome, it can be lost over time, for example, during cell division. Therefore, the therapeutic effect in the case of the use of such vectors may be temporary.
Although adenoviruses are used in gene therapy much more often, adeno-associated viruses are much more promising, due to greater safety. The fact is that when introduced into the body - adenoviruses can cause a strong immune response and an inflammatory reaction.
In the early stages of the development of gene therapy, the use of adenoviral vectors even led to the death of the patient. Jesse Jelsinger suffered from a deficiency of ornithine transcarbamylase - a genetic liver disease. At the age of 18, he took part in a clinical trial on gene therapy, and died due to a systemic inflammatory response in response to the introduction of a viral construct.
On the contrary, the introduction of adeno-associated viruses into the body only leads to a slight immune response, since in nature these viruses do not cause diseases of mammals and are hardly recognized by the human immune system.
Other viral vectors - retroviruses and lentiviruses - embed genetic material into the genome and provide a stable therapeutic effect. However, random integration into the genome can lead to the activation of cancer mechanisms, which has already happened several times in the history of gene therapy.
Retro and lentiviruses are used almost exclusively for narrowly targeted delivery to certain tissues and their areas, or to cells in a test tube, because, unlike AV and AAV, they carry material to much smaller "distances". The disadvantage of retroviruses is that they can only penetrate dividing cells. Therefore, lentiviruses are much more promising, as they infect non-dividing cells too.
In addition, non-viral methods of DNA and RNA delivery, for example, liposomes, are often used in gene therapy. They are much inferior to viral vectors in efficiency, but safer and cheaper.
 Comparative analysis of the vectors of delivery of genetic material into the cell
Comparative analysis of the vectors of delivery of genetic material into the cell
An important feature of these viruses is that they do not integrate the delivered genes into the genome. However, in the case of adeno-associated vectors, it was nevertheless shown that <1% of cases there is an integration (integration) into a certain region of the host genome.
The absence of random gene integration into the genome is a big plus in terms of the safety of these vectors. In this case, there is no risk that the gene will accidentally integrate into the sequence of the proto-oncogene and cause cancer. On the other hand, if a gene is not inserted into the genome, it can be lost over time, for example, during cell division. Therefore, the therapeutic effect in the case of the use of such vectors may be temporary.
Although adenoviruses are used in gene therapy much more often, adeno-associated viruses are much more promising, due to greater safety. The fact is that when introduced into the body - adenoviruses can cause a strong immune response and an inflammatory reaction.
In the early stages of the development of gene therapy, the use of adenoviral vectors even led to the death of the patient. Jesse Jelsinger suffered from a deficiency of ornithine transcarbamylase - a genetic liver disease. At the age of 18, he took part in a clinical trial on gene therapy, and died due to a systemic inflammatory response in response to the introduction of a viral construct.
On the contrary, the introduction of adeno-associated viruses into the body only leads to a slight immune response, since in nature these viruses do not cause diseases of mammals and are hardly recognized by the human immune system.
Other viral vectors - retroviruses and lentiviruses - embed genetic material into the genome and provide a stable therapeutic effect. However, random integration into the genome can lead to the activation of cancer mechanisms, which has already happened several times in the history of gene therapy.
Retro and lentiviruses are used almost exclusively for narrowly targeted delivery to certain tissues and their areas, or to cells in a test tube, because, unlike AV and AAV, they carry material to much smaller "distances". The disadvantage of retroviruses is that they can only penetrate dividing cells. Therefore, lentiviruses are much more promising, as they infect non-dividing cells too.
In addition, non-viral methods of DNA and RNA delivery, for example, liposomes, are often used in gene therapy. They are much inferior to viral vectors in efficiency, but safer and cheaper.
 Comparative analysis of the vectors of delivery of genetic material into the cell
Comparative analysis of the vectors of delivery of genetic material into the cell
The state and prospects of gene therapy of aging
At present, gene therapy of aging is rapidly developing, but so far it is still at the preclinical stage of development (that is, no human experiments have been performed yet). Among the 2210 gene therapy clinical trials launched to date, there is not one to treat aging. This is partly due to legal issues: aging is still not considered a disease. But this field of research itself originated very recently. The first work on gene therapy of aging in mice was carried out less than 5 years ago.
Now all research on gene therapy of aging is carried out on model mice, rats, monkeys and human cell cultures - cells in vitro.
All approaches to gene therapy of aging are divided into those where the longevity gene is delivered to the body, and those where small RNAs are introduced that "turn off" the gene or the pathway of aging. That is, in the first case, something useful is introduced for longevity, and in the second, the harmful is turned off.
In a strict sense, only two studies have been conducted on mammalian gene therapy of aging. In these studies, mice were injected with a therapeutic gene and looked at how it affected aging and longevity. The first work was carried out by the group of Maria Blasco in 2012, in which it was possible to prolong the life of mice by 20%. And the second is the treatment of the hypothalamus genome of the NF-kB inhibitor, which Donsheng Kai and colleagues conducted in the 2013th. In it, the life of mice increased by 10%. Both works are very encouraging: the therapy was very effective and without any side effects.

Much more work is modeling gene therapy on transgenic mice. In such studies, the therapeutic gene is not delivered into the body of an adult mouse, and with the help of genetic engineering they create mice whose genome has been changed since birth. Like gene therapy, it allows you to explore how an increase or decrease in the activity of different genes affects the lifespan and aging of the body.
There are so many studies where gene therapy of aging is modeled on cell lines. In such studies, it is usually considered whether the delivery of a therapeutic gene will slow down cell aging in a test tube or not. But this approach gives ambiguous results, since the therapy, which prolongs the life of the cells, does not always prolong the life of the body and vice versa. For example, the increased activity of the longevity gene and the FOXO stress response prolongs the life of model fruit flies.And in experiments on skin stem cells, cellular aging was slowed down when the FOXO3 gene, on the contrary, was turned off.
Currently, gene therapy approaches are being developed for all the major longevity mechanisms known today: increasing genome stability, rejuvenating the hypothalamus, destroying senescent cells, increasing resistance to stress, improving mitochondrial function and maintaining stem cell niches.
However, much more research is being done on the gene therapy of individual age-related pathologies, for example, age-related muscle depletion and deterioration of cognitive abilities. By themselves, they are not diseases, but they impair the quality of life and can lead to an increase in the risk of many age-related diseases.
In total, about 46 works on gene therapy of age-related pathologies were conducted on model rats, mice, and even monkeys. These are works on gene therapy in the “classical sense”: model animals (most often old ones) were injected with a gene as part of a vector for delivery. After treatment, they analyzed how this affected the occurrence of age-related pathology. Unfortunately, the vast majority of such works did not look at the side effects.
Most of the research was devoted to pathologies of the central nervous system - about 10 papers. There are 6 studies for violations of the heart and blood vessels, 7 for disorders of the reproductive system, 6 for cartilage pathologies, 4 for obesity, 7 for bone disorders, 3 for vision, 3 for the immune system, and 1 for muscles.
In conclusion, I want to note that, despite the fact that relatively few experiments on gene therapy of aging were carried out with a maximum life extension effect of 20%, it already has a huge advantage compared to other approaches to life extension (for example, geroprotectors or food, prolonging life up to 30-50%). Gene therapy is enough to hold only once in a lifetime!
For example, in the work of Andrzej Bartke in 2001, food restriction extended the life of mice by 30%. However, mice consumed a low-calorie diet for up to 670 days in a row - that is, every day, for half of their lives! And in the experiment of gene therapy Maria Blasco 2012, gene therapy with telomerase led to a slightly smaller effect - mice began to live 20% longer. However, in this work, the mice received only 1 injection of the drug into the blood in a lifetime!
 Here you need to figure out where the engineering, where the therapy.
Here you need to figure out where the engineering, where the therapy.
Therefore, if we are talking about the transmission of research on the extension of life per person, gene therapy has an absolute advantage, because it does not reduce the quality of life because of the need for constant treatment - to follow a certain diet every day or to constantly use geroprotectors or other medicines.
Telomerase Gene Therapy
The discovery of telomerase - a revolution in the study of aging
The idea to use telomerase to prolong life for more than 30 years. The discovery of telomerase in 1985 caused a real sensation among researchers of aging, and it was appreciated so highly that Elisabeth Blackburn, Carol Grader and Jack Shostak were given the Nobel Prize for it in 2004.

The history of telomerase research dates back to 1961. American researcher Leonard Hayflik cultivated human embryo fibroblasts in a test tube and noticed that they can share no more than 50 times, and then grow old. And if you take cells from older donors, then they divide even less. The scientist suggested that in the cells there is a counter of divisions, limiting their total number.
After 10 years, the Russian scientist Alexei Olovnikov offered a hypothetical mechanism for the operation of this counter. Olovnikov suggested that during cell division, the ends of the chromosomes, called telomeres, are slightly reduced. And when telomeres reach a critical length, the cell stops dividing and ages. In non-aging cells (for example, sex and embryonic stem), on the contrary, there must be an enzyme that extends telomeres, allowing the cells to divide almost indefinitely.
This hypothesis was fully confirmed with the discovery of the enzyme telomerase in 1985. Data on the role of telomeres and telomerase, not only in cell aging, but also in the aging of the whole organism, began to accumulate. In addition, it was shown that damage to the telomerase gene greatly shortens the life of model animals and leads to the emergence of premature aging syndrome - progeria.

After the discovery of telomerase, dozens of scientists caught fire in order to make a cure for old age based on it. It would seem that the "inclusion" of telomerase in all cells can make the body immortal.
However, concerns soon arose due to the fact that the active synthesis of telomerase was observed in 90% of cancerous tumors. The question arose: whether the activation of telomerase will not lead to the risk of malignant transformation? In addition, it turned out that cell aging is not always accompanied by a reduction in telomeres. For example, in the case of epithelial cells of the oral mucosa or cornea of the human eye. This suggested that telomerase activation alone may not be enough to rejuvenate the entire body.
Despite the doubts that the therapeutic use of telomerase caused, experiments began on cell lines and model animals. Dozens of studies were conducted, and the results exceeded all expectations.
Gene therapy with telomerase on cell lines
In a number of studies, telomerase activity was activated in human cells in vitro (in vitro). Such works, on the one hand, allow us to learn more about the properties of telomerase, and on the other hand, they are the first step in the development of a life-prolonging drug.
To do this, the telomerase catalytic subunit (TERT) gene is inserted into the cell DNA. It is she who carries out the lengthening of telomeres in the cell.

The first successful study on the delivery of the TERT gene was conducted in 1998 by Andreai Bodnar and his colleagues. It turned out that with the activation of telomerase, human fibroblasts, which normally divide no more than 75–80 times, are able to share 280. At the same time, they do not have signs of aging, pathology or malignancy (cancer transformation). Even if such cells are transplanted to nude mice - completely deprived of immunity and more susceptible to cancer - they still do not have tumors.
In subsequent years, dozens of studies were conducted in which the telomerase gene was introduced into a wide variety of human cell types. Delivery of the TERT gene to dividing cells increased their potential for proliferation, and delivery to old cells led to "rejuvenation" and the resumption of divisions.
Successful experiments were conducted on mesenchymal stem cells, bone stem cells (osteoblasts), myosatellites (precursors of muscle tissue), articular chondrocytes (precursors of cartilage tissue), and also on cells of the intervertebral disk, T lymphocytes, hepatocytes (liver cells), and cells olfactory bulbs, etc.
It is important to note that in most cases the delivery of the telomerase gene was absolutely safe. The cells retained their functions, and no signs of tumor formation were observed. However, in some studies, the introduction of the TERT gene led to an increase in the frequency of chromosomal rearrangements (chromosome breaks, sticking, movement of sections of one chromosome to another). This, of course, is a risk factor for cancer, but it may not have negative consequences.
In general, research results indicate that the work of telomerase in human cell culture significantly slows down aging and does not necessarily cause the development of cancer. That is, telomerase is devoid of the properties of oncogene, which was attributed to it. Apparently, the main property of telomerase is the regulation of cell division, and additional mutations and factors are necessary for the onset of tumor growth.
Telomerase Gene Therapy: From Experiments in Mice to Man
For the first time, experiments on gene therapy of aging were conducted in 2012 by the Spanish researcher Maria Blasco using the telomerase gene (TERT). The first results were very, very impressive. Gene therapy not only adults, but also old mice prolonged life up to 20%! If we imagine that such results can be achieved on a person - we will save 14 years of human life!
Before proceeding to gene therapy, the effects of telomerase were investigated in transgenic mice. It turned out that if you "turn on" the TERT gene in all cells of the mouse, then the lifespan is increased by 40%! However, the constant activity of telomerase increased the risk of cancer.
Therefore, there was a question about how to activate telomerase work for a shorter period.
That is what was done in the work of Maria Blasco 2012. The telomerase gene was delivered to the mouse using an adeno-associated virus (AAV9) capable of providing systemic delivery. Adeno-associated viruses are characterized by high safety: they do not embed the delivered gene into the host genome, and therefore do not lead to mutagenesis. In addition, they almost do not cause an immune response.
The telomerase gene was delivered to a wide range of tissues and organs, including the liver, kidneys, lungs, heart, brain, and muscles. Telomerase was found in these tissues even 8 months after the procedure. In animals, a number of age parameters have improved: insulin sensitivity has increased, neuromuscular coordination has improved, the risk of osteoporosis (bone depletion) and the content of molecular markers of aging have decreased. In addition, TERT gene therapy was completely safe: the risk of cancer in mice did not increase.
And, most importantly, gene therapy significantly prolonged the life of the mice. The median life expectancy (the age to which more than half of the individuals in the group live) increased in adults (1-year-olds) and old (2-year-old) mice by 24% and 13%, respectively. The maximum lifespan also increased: by 13% in adults and by 20% in old mice.
In the next study, Maria Blasco showed that the delivery of the TERT gene to the heart of mice after myocardial infarction significantly improves heart function and reduces the risk of heart failure and mortality by 17%.
Thanks to these experiments, telomerase gene therapy has a brilliant reputation. And in September 2015, the world's first experiment on human gene therapy of aging was conducted. The American Elizabeth Perrish introduced herself a telomerase gene in combination with the myostatin inhibitor gene (to stimulate muscle growth). This study has just begun, so it's too early to talk about the results, but for now Elizabeth feels good. Elizabeth pledged to provide analysis material to any medical science institution upon request. However, since only one person participated in this experiment, its results will not allow making reliable, statistically significant conclusions.
However, the fact that human trials have begun is evidence that the TERT-based medicine is already on the way. Without a doubt, it is the number one candidate to fight aging with gene therapy.
Gene Therapy for Hypothalamus Rejuvenation
One of the ways to prolong life is to narrowly influence the structures that regulate the aging of the whole organism. Studies in recent years show that this structure is the brain section - the hypothalamus.
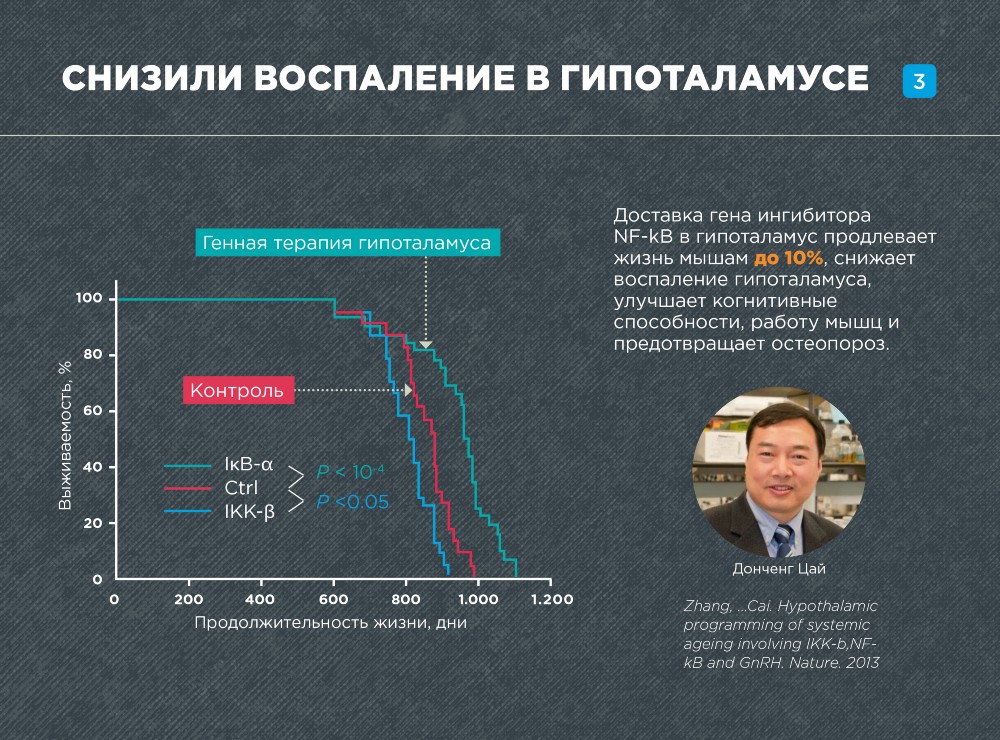
In 2013, American scientists under the leadership of Dongsheng Kai significantly extended the life of mice, rejuvenating the hypothalamus with the help of gene therapy. Researchers suppressed the inflammation arising with age in the hypothalamus, as a result of which the number of age-related pathologies decreased, and the life expectancy increased by 10%.
Aging is largely associated with impaired homeostasis - the body's self-regulation, its ability to maintain its internal state. In the "young" state, the body reacts to an imbalance effect by a set of measures that successfully return it to its original, "healthy" state. For example, with an increase in body temperature, the body starts a series of thermoregulation processes (for example, perspiration), as a result of which the temperature decreases to its original level. However, with aging, the ability to maintain homeostasis is impaired, which leads to an "imbalance" of the body.
The hypothalamus is one of the main centers of the body that regulate homeostasis. Despite the fact that it makes up no more than 5% of the brain, it is he who links the nervous and hormonal mechanisms for maintaining homeostasis into a single system.
With age, the work of the hypothalamus greatly deteriorates, in particular, decreases the production of the most important hormone - GnRH. The “classic” function of this hormone is to regulate sexual function. But for aging, it plays a secondary or even a minor role. It is important that GnRH also activates neurogenesis - the formation of new nerve cells in the hypothalamus, as well as in a number of other areas of the brain.
One of the main causes of age-related impairment of the hypothalamus is inflammation. With age, the transcription factors NF-kB and IKK-b, the regulatory molecules that trigger the work of a whole cascade of genes responsible for inflammation, are activated in the hypothalamus.
To rejuvenate and reduce inflammation in the hypothalamus, Donsheng Kai and his colleagues used a gene that inhibits NF-kB - IkB-a. The IkB-a gene was administered to adult mice by injection into the hypothalamus. In order for the gene to penetrate into the cell nuclei and integrate lentiviral vectors into the genome.
The therapy has a very strong effect on aging. The maximum lifespan of mice increased by 10%! The health of the mice also improved significantly. Even 6 months after the injection, the mice had better mental performance, muscles worked better. An analysis of the tissue structure also showed that the muscles, skin and bones of the mice were in a more “young” state.
Thus, the hypothalamus is an important center of regulation for systemic aging. Its rejuvenation leads to the prolongation of the life of the organism, apparently, both by improving its own regulatory functions of the hypothalamus and the release of sex hormones, and by stimulating neurogenesis and, as a result, improving the work of other brain regions.
Gene therapy to increase stress resistance
Among the ways to extend life, studied to date, the most powerful is to increase stress resistance. It is due to the effects on this path that all current life extension records are set. Robert Schmukler-Rice managed to extend the life of nematodes 10 times, and Andrzej Bartke the life of a mouse - 1.8.
Throughout life, the body constantly faces a variety of damaging factors: hunger, thirst, cold, toxic substances, damaging radiation, psychological stress. Resistance to these factors and got the name - stress resistance.
Increased stress tolerance can occur at all levels: from molecules to the whole organism. However, mainly molecular mechanisms have been studied. Increased stress tolerance is accompanied by an increase in protein stability; if there are already violations in the structure of protein molecules, then they are corrected, and the cell gets rid of completely destroyed molecules — it is broken down into pieces and digested.
Increased stress resistance can be achieved in several ways.

The first, most well-known method is to restrict nutrition. At the same time, the work of the MTOR complex is “turned off” MTOR is a kind of molecular “switch”. It translates the cell into a state of active growth and reproduction. At the same time, energy-intensive stress-resistance mechanisms are suppressed. If the mTOR is “turned off”, the cell, on the contrary, will go into protection mode against stress.
The second, also well-studied, way to increase stress tolerance is to block insulin signaling and insulin-like growth factor 1 (IGF-1). By signaling (or signaling) we mean a cascade of molecular processes occurring in a cell in response to the action of hormones. Insulin and IGF-1 signal the cell to the presence of nutrient glucose in the blood. As a result, growth processes are triggered in the cell, the MTOR pathway is activated, and stress resistance processes are suppressed.
Inhibition of the MTOR or insulin pathway - triggers a whole cascade of stress response genes that simultaneously turned out to be longevity genes: FOXO, NRF2, AMPK, HIF-1, SIRT1 and SIRT6, etc. Thus, the third way to increase stress resistance is artificial activation stress response genes.
All of these approaches are being actively studied for gene therapy of aging. In some works, the hypothalamus, the structure that regulates aging, is affected separately; in others, it is systemic on most cells of the body. There are very few real works on gene therapy, where a therapeutic gene could be obtained in an adult animal, more precisely, only one. In most studies, gene therapy is modeled on transgenic animals or cell lines (on cells in vitro).
Activation of the neuropeptide Y gene in the hypothalamus
The first and only work on gene therapy to increase stress resistance was carried out on mice less than a year ago. It affected the regulator of systemic aging - the hypothalamus.

Luís Pereira de Almeida, Janete Cunha Santos and Cláudia Cavadas
A group of Portuguese and German scientists led by Luís Pereira de Almeida and Cláudia Cavadas in 2015 conducted an experiment where they activated the neuropeptide Y (NPY) gene in the rat hypothalamus.
Neuropeptide Y is one of the most important signal molecules of the hypothalamus. In recent years, much data has appeared on the role of this substance in aging. For example, the work of the NPY gene is necessary in order for calorie restriction to prolong life. And in transgenic rats, which produced more of this neuropeptide, even the median life expectancy increased (the age to which half the individuals in a group live).
It is amazing that a few years ago not a word was known about the role of NPY in aging. And neuropeptide Y was mentioned only in connection with its role in obesity and the ability to stimulate the feeling of hunger. It is surprising how these functions combine with the ability to prolong life!
Scientists delivered the NPY gene using an adeno-associated virus to the hypothalamus. As a result, the most important mechanism of resistance to stress - autophagy (the process of self-purification of cells from damaged molecules and cellular structures by their digestion) was stimulated in the cells of the hypothalamus.
No other effects of NPY genome therapy on longevity have been studied in this work, but researchers are going to do this in the future.
However, it can already be assumed that the therapy invented by de Almeida and Cavadas will slow down aging by rejuvenating the hypothalamus (a regulator of systemic aging). On the other hand, it is possible that neuropeptide Y stimulates autophagy not only in the hypothalamus, but also in other cells of the body, which will also lead to a delay in aging.
Activation of the UCP2 gene in the hypothalamus
In 2006, American scientists under the guidance of Tamás Bártfai conducted another elegant experiment, where they also worked on the hypothalamus. Exposure resulted in a slight decrease in body temperature and, unexpectedly, increased longevity!
This was not a gene therapy experiment — transgenic mice whose genome was modified from birth were used. But, given the effectiveness of current methods for the delivery of genes into the body, the translation of these studies into gene therapy presents no particular problems.
Transgenic mice in Tamás Bártfai-I experiments produced an increased amount of uncoupling protein 2 (UCP2) in the hypothalamus. This is a mitochondrial protein that, when used, reduces the efficiency of ATP synthesis and promotes heat release. As a result, the temperature of the hypothalamus increased slightly. And the increase in temperature in the main body thermostat led to the fact that the overall body temperature slightly decreased - by 0.3 ° - 0.5 ° C.
A slight decrease in body temperature caused an effect in mice that was similar to the effect of food restriction, despite the fact that mice ate as much as they wanted. Moreover, the median lifespan of mice significantly increased: in males by 12%, and in females - by 20%.
In other works, stress resistance was increased, acting systemically on most cells of the body.
Initiation of the Pathway of Aging MTOR
Toren Finkel and his colleagues increased the stress tolerance and longevity of mice, “turning off” the most famous way of aging, MTOR.
In the experiments, transgenic mice with a mutation at the mTOP locus (regions on the chromosome where the corresponding genes are located) were used, which had 75% less mTOP protein in the cells. It turned out that these mice live 20% longer.

In addition, the mutation in the MTOR locus significantly improved many of the health indicators of mice: a number of organs and tissues were more “young” and in good condition, there were fewer protein aggregates (adherent proteins) and damage in the tissues. In addition, the memory, coordination, and spatial thinking of these mice worked better.
However, the "shutdown" of the MTOR pathway was accompanied by side effects: the condition of the bones deteriorated, mice more often suffered from infectious diseases of the mouth, skin and eyes. In addition, the mice were smaller. Thus, the long-term shutdown of the mTOR complex genes seems to be quite traumatic for the body, and one should either reduce the inactivation time of the mTOP or combine it with another effect that compensates for negative side effects.
Activation of the PTEN gene
Another promising target for gene therapy is the PTEN gene - it "turns off" the insulin pathway of aging. PTEN inhibits the operation of phosphatidylinositol-3-kinase type I (PI3K) - a molecule necessary for the transfer of insulin signal into the cell.
Spanish scientists headed by Manuel Serrano investigated transgenic mice with overexpression (increased activity) of the PTEN gene. Such mice were much better protected from cancer, and their maximum lifespan was 16% longer. Transgenic mice were more sensitive to insulin, protected from diabetes and steatosis - a pathological accumulation of fat in the liver, they had less damage to DNA in old age.

Another mechanism was discovered in addition to the inactivation of the insulin pathway, by which PTEN affects longevity. Activation of PTEN led to the fact that the brown adipose tissue began to release a lot of energy in the form of heat, which caused an increased expenditure of energy of the whole organism. And, as is known from earlier works, life expectancy is directly dependent on the intensity of energy consumption.
"Turning off" the AC5 gene
Another, less well-known way to increase stress resistance is to block the transmission of a signal from the stress hormone - adrenaline into the cell. Adrenaline is a hormone that causes a flight or fight reaction ("flight or fight"); he begins to actively stand out with a sense of danger, fear, trauma, burns, shock and in border situations, mobilizing the body to eliminate the threat. At the same time, adrenaline is a very powerful stress hormone, with prolonged exposure, it damages cells and whole tissues, leading to depletion of the body.
The American researcher Stephen F. Vatner and colleagues created transgenic mice with the “turned off” gene of adenylate cyclase type 5 (AC5), the molecule necessary to transfer the adrenaline signal to cells through one of its receptor types, β-adrenoreceptors. It is important to note that such receptors are present on almost all cells of the body.
The “switching off” of the AC5 gene unexpectedly had a very strong effect on health and longevity. Transgenic mice lived by as much as 30% longer than normal mice and were less prone to bone and heart pathologies.
At the molecular level, these effects were due to the fact that the inactivation of AC5 launched the path of resistance to stress - Raf / MEK / ERK. As a result, the cell produced a whole set of protective molecules, triggering the mechanisms of cell survival.
In addition, it is known from earlier works that the action of adrenaline on a cell through β2 -adrenoreceptors directly causes DNA damage. Thus, it can be assumed that "turning off" the AC5 gene of mice also contributed to increased genome stability.
Activation of sirtuins genes
Other important participants in stress tolerance and longevity mechanisms are sirtuins. Especially sirtuins 1 and 6 (SIRT1 and SIRT6) have been studied. Sirtuins are histone deacetylases - they change the DNA packing density and inhibit the expression (decrease activity) of genes.
Histones are molecules that provide spatial packing of DNA: DNA is wound on them like a thread on a coil, and the coils are tightly packed. If the coils are packed too tightly, it becomes almost impossible to read the genetic information from them. Deacetylases increase the packing density of DNA, making it inaccessible for reading. As a result of the activation of sirtuins, the expression of a whole set of genes is inhibited.
Sirtuin 1
Sirtuin 1 regulates a number of longevity processes: it suppresses inflammation, promotes cell survival, triggers the division of cell power stations - mitochondria, and activates the most important stress tolerance and longevity gene - FOXO. Several studies on transgenic mice have shown that activation of the SIRT1 gene has a positive effect on health. In the first study of 2010, conducted by Spanish scientists led by Manuel Serrano, the SIRT1 gene was activated throughout the body of mice. As a result, their cells grew slower, there was less DNA damage and less often cancer developed.

After 3 years, Shin-ichiro Imai from the University of Washington activated the SIRT1 gene not in the whole body, but only in the brain of mice, which led to a significant extension of their life. Transgenic females lived 16% longer, and males - 9%.
In addition, to date, many works have been carried out, where therapy with the SIRT1 gene has been modeled on human cell lines. Delivery of the SIRT1 gene rejuvenated and stem and differentiated (specialized in function) human cells increased the number of divisions and slowed the onset of cell old age.
Sirtuin 6
Another major participant in longevity processes is sirtuin 6 (SIRT6). It prevents inflammation, modifies signals from insulin-like growth factor 1 IGF1, activates DNA repair pathways and some other mechanisms of genome stability.
Increased expression of the SIRT6 gene contributes to longevity, as was shown in 2 studies on transgenic mice.
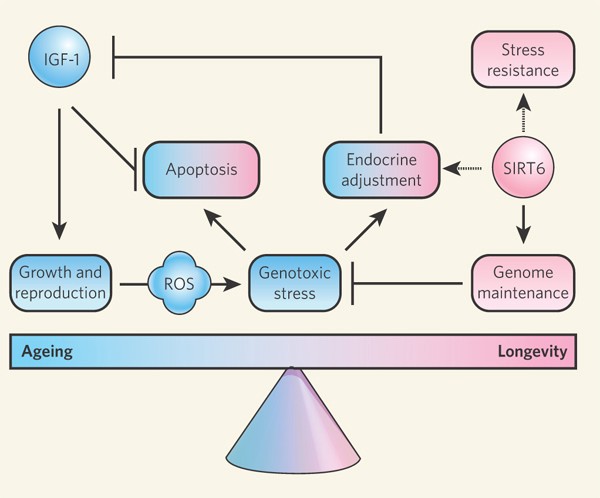
In 2012, American and Israeli scientists, led by Haim Y. Cohen, increased the expression of SIRT6 in the whole body of mice, which extended the lives of males (but not females) by 10–14.5%. Surprisingly, despite the longevity, transgenic mice do not have less age-related diseases, but even with diseases such as cancer, transgenic mice have lived much longer. A few years later in the work of the same scientists (according to unpublished data) activation of the SIRT6 gene increased the life of not only males, but also females. However, it should be borne in mind that the activation of SIRT6 is not useful in all tissues. In the brain, its increased production, on the contrary, predisposes to neurodegenerative diseases.
A number of successful studies on the treatment of the SIRT6 gene on human cell lines have been carried out. It is shown that its delivery rejuvenates the cells of the lining of the bronchi and blood vessels and cartilage tissue.
Interestingly, SIRT6 gene therapy is not yet used for aging, but is already used for the age-related pathology of cartilage - osteoratrosis. Studies have been conducted on gene therapy in both cells and mice. After the introduction of the SIRT6 gene into the knee, the cartilage functioned much better, and much less pathological changes occurred in it.

NRF2 gene activation
An alternative approach to increasing stress tolerance is to influence the transcription factor NRF2. This pathway is highly active in the cells of long-living animals: the bare excavator and the dwarf Snell mice.
NRF2 is a transcription factor - a molecule that regulates the activity of hundreds of other genes. It begins to work in response to toxic effects and launches more than 200 genes that protect the cell from damage, inflammation and increase the stability of proteins.
This path was immediately adopted by scientists involved in the treatment of neurodegenerative diseases (in particular, Alzheimer's disease), and have already done several work on gene and cell therapy in mice.
Many researchers tend to treat neurodegenerative diseases with the help of stem cell delivery to the brain. However, with such diseases in the brain, there is a high level of oxidative stress, and stem cells die during injection. And the pre-delivery of the NRF2 gene makes such cells more resistant to stress, so that after introduction into the brain they do not die.
In other studies, the NRF2 gene was delivered directly to the brains of Alzheimer's mice. Introduction of the NRF2 gene into the hippocampus (brain structure responsible for memory) improved memory and spatial learning. In another work, delivery of the NRF2 gene to the brain made it more resistant to oxygen starvation (hypoxia) and improved the mental abilities of mice.
In general, to increase stress tolerance, the most research on gene therapy is conducted! All the main participants of the signaling pathways are checked - molecules that inhibit the aging pathways of IGF-1 and mTOR, as well as those involved in various stress tolerance ways. It is the increase in resistance to stress that leads to the maximum effect of life extension in transgenic mice - up to 1.8 times. However, the “classic” work on gene therapy of stress resistance (where a therapeutic gene is delivered to the body of a model animal) is extremely small, or rather only one. But when scientists move from experiments on transgenic animals to gene therapy, there is every chance of expecting unprecedented success in the field of life extension.
Gene therapy to destroy old cells
Another strategy for extending life is the destruction of old - senescent - cells. Old cells accumulate in the body with age and contribute to the aging of the whole organism. Especially many senescent cells are found in the foci of age-related pathologies - ulcers, atherosclerotic plaques, diseased joints, and brain in Alzheimer's disease.
Cells become senescent when they lose the ability to share and perform their functions. Having become sensational, they begin to secrete a whole bunch of harmful substances (SASP), which violate the structure and functions of neighboring tissues, and also contribute to the cancerous transformation of cells.
Apparently, the evolutionary formation of such cells in the body arose as a defense mechanism against cancer. In order not to turn into cancer cells, damaged cells lose the ability to divide, moving to a special state called the senessens. And the substances they excrete (SASP) signal to other cells that damage has occurred, and stimulate tissue repair and wound healing.
According to Judy Kampizi, a researcher at the Baka Institute (California), cell aging is a phenomenon that is beneficial in youth, but very harmful in old age. In youth, the emergence of a small number of such cells protects the body from cancer, and the substances secreted by them contribute to changes in the structure and repair of tissues. However, the accumulation of a large number of such cells in old age leads to the fact that the factors they emit become too much. This translates into chronic inflammation, which, in turn, contributes to all age-related pathologies, ranging from neurodegeneration to cancer.
For the first time, American researcher Jan M. van Deursen applied the destruction of senescent cells to slow down aging. The study was published in 2011 in the best world journal Nature. Experiments were performed on transgenic mice with premature aging. A suicidal gene was inserted into the genome of such mice, which began to work only in old cells. In the remaining cells, it remained inactive. It was possible to cause the death of senescent cells in such mice at any time by introducing the substance AP20187 to mice. An enzyme encoded in the suicidal gene made the substance toxic, which killed the senescent cells.
The results showed that if you destroy senescent cells all your life as they arise, then age-related pathologies (adipose tissue, muscles, eyes, and many other organs) occur in mice much later. However, one can begin to destroy old cells in old organisms, when all these pathologies have already arisen.In this case, the pathological condition is much easier.
Since that time, the research group Jan M. van Deursen has made significant progress in their research. At the beginning of 2016, they published an article, again in the journal Nature, where they showed that the destruction of senescent cells slows down aging in normal mice with normal (not accelerated) aging. Therapy slowed down the occurrence of cancer, prevented the occurrence of pathologies of the heart, kidneys and adipose tissue, and, moreover, prolonged life by 17–35%!
Gene therapy to improve mitochondria
With age, there is a strong deterioration of the mitochondria, which contributes to the aging of the cells and the whole organism.
Mitochondria are cellular power stations, they provide the cell with the main energy. “Cellular respiration” occurs in them - the generation of energy due to the oxidation of nutrients and its storage in the form of high-energy ATP molecules.
Particularly interesting is the origin of mitochondria. They are descendants of bacteria swallowed by primitive cells 1.5–2 billion years ago. During the time of existence inside the cell, the mitochondria were greatly simplified, they became dependent on the cell, but they still retained the features of independence. For example, mitochondria have their own DNA, as well as their own molecular “machines” for the synthesis of RNA and protein.
With aging, the number of mitochondria and their quality decreases. In the process of “cellular respiration,” mitochondria begin to release much more by-product — the active forms of oxygen that damage the cell and also produce much less energy. As a result, the cell does not have enough energy to carry out the processes of repair and regeneration.
Currently, only one approach to mitochondrial gene therapy in old age is being actively studied - activation of the PGC-1 gene. This gene is the most important regulator of the number of mitochondria in mammals, as well as the longevity gene.
Drosophila showed that its overexpression in intestinal cells prolongs life by 33–37%. In humans, the PGC-1 gene is involved in the occurrence of a number of age-related pathologies. Deficiency of PGC-1 contributes to neurodegeneration and type 2 diabetes, and the introduction of PGC-1 restores the kidneys to a field of damage.
The PGC-1 gene is not yet used directly for the therapy of aging, but it is actively used to treat various age-related pathologies. Several works have been carried out, both on cages and on model mice.
Chinese researchers, led by Wei-Jia Kong, used the PGC-1 gene for deafness therapy on a cell model. The introduction of this gene slowed down the aging of the rat cells responsible for the perception of sounds. In another paper, the Chinese scientist Bing Chen and his colleagues successfully used the delivery of the PGC-1 gene to stem cells to increase their resistance to stress. And Pei-Qing Liu, introduced the PGC-1 gene into the cells of the heart, which protected them from hypertrophy (pathological growth).
Several experiments have already been carried out on mice. Scientists from Boston, led by Zolt Arany, delivered the PGC-1 gene to older mice with peripheral arterial disease. The therapy successfully restored the blood supply to the muscles. And researchers Hollinger and Selsby used PGC-1 gene therapy for the treatment of a hereditary muscle disease - Duchenne myodystrophy, which, however, is not age-related. However, the therapy was successful in this case too: the delivery of the PGC-1 gene significantly improved the functioning of the muscles in mice with pathology.
Gene therapy to maintain genome and epigenome stability
A characteristic feature and, apparently, one of the main causes of aging is the accumulation of DNA damage. In addition, numerous diseases of premature aging - progeria - also arise as a result of excessive accumulation of genomic damage.
Therefore, one of the most promising ways to prolong life is to repair damage and increase the stability of the genome.
During the life of the DNA is constantly damaged by various effects. It is affected by temperature, UV radiation, chemical mutagens and viruses from the external environment. But the internal processes of the cell itself increase the genomic instability: DNA is damaged by active forms of oxygen from mitochondria, and replication systems (DNA doubling) and repair often do not work accurately and introduce errors themselves.
As a result, with age, a whole spectrum of disorders accumulates in the DNA: breaks, point mutations, transfer of pieces of DNA from one place to another, sticking of chromosomes, inserting viruses and transposons into the genome. If there is too much damage, the cell is forced to go one of the 3 ways: start the processes of cell suicide (apoptosis), become sensitized, or turn into cancer. All these processes (if they are subject to many cells) are extremely harmful to the body.
The most studied way to increase the stability of the genome is the activation of DNA repair systems. This approach is used in most of the work on gene therapy.
Activation of DNA repair
DNA reparation is the most important task for the cells, since it allows you to save the most valuable information contained in the DNA. It is not by chance that in the evolution of the cell, several complementary systems for the detection of DNA damage and their “healing” were formed. These are, for example, recognition pathways for 2- and 1-strand DNA breaks; various DNA repair systems - excision repair, which allows to remove damaged nitrogenous bases from DNA, non-homologous compound of the ends and homologous recombination, which heals 2-strand DNA breaks, replication activated replication, etc. These systems involve hundreds of proteins whose functions are intertwined with a complex and bizarre network . Potentially, each of these genes is a candidate for gene therapy of aging, however, to date, research in this area is just beginning.

In 2013, Italian scientists led by Gemma Calamandre investigated transgenic mice with the activation of the MTH1 gene. Protein MTH1 is involved in the repair of DNA damage and RNA caused by reactive oxygen species. It corrects defective nucleotides - guanidines oxidized at the eighth carbon atom (of the four letters on which this DNA is written is denoted by “G”).
It turned out that increased expression of MTH1 prolongs the life of transgenic mice by up to 22%. In addition, in such mice, genomic lesions did not accumulate with age. Their behavior also changed: the mice became less disturbing and more curious.
In another study, Johannes Grillari and colleagues conducted gene therapy on another repair protein, SNEV, on cells in vitro. SNEV "patches" DNA breaks, and also participates in the destruction of damaged proteins and cleansing the cell.
Delivery of the SNEV gene into the cells of the human lining of the vessels (endothelium) of a person slowed down their aging significantly: the cells were divided 2 times more before they grew old and stopped dividing. In addition, they become more resistant to stress.
Suppression of retrotransposon activity
DNA damage can also be caused by its own genomic sequences - retrotransposons. These are the remnants of the DNA of retroviruses, which in ancient times were integrated into the genome, and now occupy about 42% of the human genome!
Retrotransposons are usually inactive in cells, however with age they are activated - and RNA begins to be read from them. This leads to DNA damage and chromosomal rearrangements, and also - can cause an autoimmune response, since the cell decides that it is infected with viruses. All these processes contribute to cellular aging.

In 2011, Victoria Luniak of the Buck Institute, USA, conducted a study on gene therapy, where she suppressed the activity of retrotransposons in old human stem cells. To do this, lentiviruses were introduced into old cells, from which small RNAs (shRNA) were read, which suppress the reading of information from retrotransposons.
Gene therapy was successful and led to the rejuvenation of old cells - they began to divide again.
Increased epigenome stability
To prolong life, it is extremely important to maintain stability not only of the genome, but also of the epigenome. The fact is that with age there are changes that do not affect the DNA sequence, but, nevertheless, change the activity of genes. Such changes are called epigenetic. Their peculiarity is that they can not only persist in a series of cell divisions, but also be transmitted to the next generations.
There are about 18,000 genes in the human genome, but not all of them work in cells. The cells of the vast majority of tissues have a practically identical genome, not counting a certain number of mutations that arise as the organism develops. However, differences in the structure and functions of different tissues are not determined by these minor deviations. The fact is that the cell of each tissue has a very specific set of genes that work and which are “turned off”.
In part, this set - the gene expression profile - is determined by the work of numerous proteins (for example, activators and repressors of the work of genes). But to a large extent it is associated with the epigenetic state of the cell (mainly the density of DNA “packing”). Therefore, maintaining stability of the epigenome is a process of extreme importance!
With age, however, epigenetic changes occur that violate the profiles of the genes, which negatively affects cellular processes and accelerates cellular aging. For example, with age, a whole set of genes and genomic sequences are activated, which normally should not work (for example, retrotransposons).
Gene activity is regulated by many factors. One of them is the density of DNA “packing”, which determines its availability for enzymes carrying out RNA synthesis. DNA in the nucleus is always in a "packed", wound on proteins - histones - condition. Like thread on the spool. If the gene is “packed” too tightly, it is unavailable for enzymes, and RNA synthesis does not occur. If the "packaging" is loose - the enzymes easily sit on it and begin to work.
Activation of genes during aging is precisely due to the fact that the density of DNA “packing” decreases.
In this regard, several researchers have decided to develop a gene therapy that globally increases the density of DNA “packaging” in the cell.
Stephen L Helfand and colleagues activated the Suv39 methylase gene, an enzyme that modifies histones by increasing the DNA packing density. The experiments were carried out on fruit flies - fruit flies, which as a result of the impact began to live longer.
And Canadian scientists Igor Kovalchuk and Olga Kovalchuk used the same approach, but on human cells. In their work, they found that the activity of the Suv39h1 gene decreases in old age. They introduced the Suv39h1 gene into human fibroblast cells (connective tissue), which led to their rejuvenation and stimulated division.
It is necessary to understand that such an approach is an extremely gross impact on the epigenome. Surely, the slowing down of aging requires a much more "jewel" effect and epigenetic changes of a narrow set of certain genes. However, for now, knowledge of the role of epigenetics in aging is very schematic and approximate.
Currently, gene therapy to increase the stability of the genome and the epigenome is an extremely young area. Most of the work was carried out not even on transgenic animals, but on cells in vitro, and the progress achieved is rather restrained. However, it is necessary to understand that while fundamental research has affected only a small layer of what actually happens in old age with the genome and epigenome and revealed very few (rather rough) ways how to counteract it.
However, this topic is extremely promising and certainly holds many surprises and has great potential for gene therapy!
Gene therapy to activate the hormone klotho
Almost 20 years ago, in 1997, Japanese and American scientists, led by Yo-ichi Nabeshima, discovered a new longevity gene and named it in honor of the Greek goddess of fate, the weaving thread of life - Klotho. His "off" in transgenic mice caused accelerated aging syndrome. And after 7 years, the same researchers found that the activation of klotho significantly prolongs the mice’s life - by males by 20–30%, and by females - by up to 19%.
 And in 2014, it was shown that certain variants in the klotho gene (SNP Rs9536314) are associated with longevity and high mental abilities in humans.
And in 2014, it was shown that certain variants in the klotho gene (SNP Rs9536314) are associated with longevity and high mental abilities in humans.
Klotho is produced in the kidneys and blood vessels of the brain and is carried by the blood through the body like a hormone. It acts systematically on most cells in the body - binds to receptors on their surface and inhibits insulin and IGF-1 signaling (one of the main ways of aging).
Three years after the discovery of the klotho gene - in 2000 - the first work on gene therapy was carried out, though not from aging, but from chronic vascular disease - atherosclerosis. Ryozo Nagai and colleagues systematically introduced this gene to rats and achieved improved vascular status and reduced pressure.
Since then, about 4–5 successful works on klotho gene therapy in model rats and mice have been carried out. True, all of them are devoted not to aging, but to various age-related pathologies of the cardiovascular system and kidneys. This increased pressure - hypertension, kidney disease, coronary artery disease and diabetes.
Gene therapy for age-related pathologies
In old age, the condition of almost all tissues and systems of the body worsens - the vascular and heart tone decreases, thymic degeneration - the most important organ of the immune system - decreases, bone, cartilage and muscle tissues are exhausted, numerous cognitive impairments occur - and this is only the beginning of the list.
Most of these pathologies are not considered diseases, but very significantly increase their risk. For example, high blood pressure - hypertension - is a powerful risk factor for stroke, heart attack and heart failure. In addition, in old age, these pathologies develop in a comprehensive manner, which significantly reduces the quality of human life and leads to decrepitude.
It is with such disorders that gene therapy of age-related pathologies fights.
However, unlike gene therapy of aging, it is always limited to any single tissue or system - the treatment of obesity, retinal degeneration, or abnormalities of the memory center - the hypothalamus. Gene therapy of aging, on the contrary, seeks to rejuvenate all the cells and tissues of the body without exception.
And if gene therapy of aging affects conservative pathways of aging, common to most cells of the body, gene therapy of age-related pathologies is usually limited to effects on mechanisms specific to a particular tissue. Quite often, these are “secondary” or “tertiary” paths, regulated by deeper and more fundamental mechanisms of longevity.
It is clear that treating age-related pathologies is much easier than aging — for this, there is no need for in-depth knowledge of the molecular mechanisms of aging. Therefore, it is not surprising that gene therapy for age-related pathologies is a much more developed area. If no more than 2–3 works on gene therapy in mammals are carried out for aging, then for age disorders - more than 40!
The purpose of such therapy is to return the old tissue or system to a state indistinguishable from young animals. Naturally, it is impossible to cope with aging in general. If the patient is rescued from a heart attack - after some time he will develop, for example, diabetes! There are even studies that show that if we could completely cure all diseases of cancer (and this is one of the main causes of death), this would extend the average life expectancy of people by only three years. So, in general, this approach is much less effective than gene therapy of aging, rejuvenating many systems at the same time and, in addition, prolonging life.
Nevertheless, quite a lot of high-quality work on model animals — mice, rats, rabbits, and even monkeys — has been done on gene therapy for age-related pathologies. This is gene therapy in the “classical sense”: a therapeutic gene is introduced into model animals for delivery and an analysis of how this will affect the occurrence of age-related pathology. At the same time, the value of the research increases significantly if old animals are used. We tried to analyze just such work.
The developed approaches are a powerful means of preventing diseases and treating senile senility. However, it seems that it makes sense to apply them only if the patient has a clear predisposition to one or another type of diseases - cardiovascular, neurodegenerative, etc.
Cardiovascular system
Gene therapy for age-related pathologies of the cardiovascular system was carried out for about 7 works on old mice and rats. This is one of the most exciting areas - because today cardiovascular diseases are the main cause of death in the world, they account for about 30% of all deaths - 17.3 million people annually. However, the occurrence of these diseases is preceded by various pathologies that can be diagnosed and treated - this is a violation of the formation of new blood vessels, a decrease in muscle tone of the blood vessels and the heart, and hypertension. They were chosen as targets for gene therapy.
In most of the work on gene therapy, they used very specific genes that improve only the work of the heart and blood vessels, none of which was at the same time the longevity gene. For example, for the treatment of high blood pressure - hypertension - the BNP (B-type natriuretic peptide) gene was delivered to the body, which is secreted by the heart during an increased load and dilates blood vessels, stabilizing blood pressure. And for senile pathology of the heart - loss of the ability to relax - used the calcium pump gene, pumping calcium from the cytoplasm of muscle cells and relaxing muscles.
All these approaches were successful, but did not lead to a radical rejuvenation; besides, in one work side effects were not investigated.
The most interesting study without a doubt is the work of the French scientist Alain-Pierre Gadeau, where the gene of the embryonic signaling cascade - the desert hedgehog (dh) - was used as a therapeutic agent. In addition to the important role in embryonic development, this gene is also "turned on" after ischemic damage (with a lack of blood supply), ensuring the formation of new blood vessels. The introduction of this gene to old mice after ischemia was very successful - the blood supply, as well as the density of the nerves in the limbs of old sick mice, became the same as in young!
Osteoporosis
About 7 studies were carried out on osteoporosis gene therapy - bone depletion in old age, leading to fragility and fractures. However, only two of them were carried on old animals. In the others, osteoporosis was induced artificially.
In all studies, bone-specific mechanisms were chosen as targets. For example, delivery of BMP genes promoting bone formation was used in 2 studies. And in several other studies, osteoprotegerin (OPG) or omentin 1 genes were delivered to prevent bone destruction. The effectiveness of all these approaches was average — the state of the bone tissue improved, but only partially; and side effects were not investigated.
Perhaps the only exception was the work of American scientists under the leadership of Xiao-Bing Zhanga - on gene-cell therapy of osteoporosis. Scientists delivered the gene PDGFB - a growth factor that stimulates bone formation - into the stem cells of a mouse - after which the cells were introduced into the body. It turned out to be extremely effective: the bone structure improved, and the strength increased by 45%! It is important that in this work investigated the side effects - and found nothing. However, all experiments were carried out on adult, healthy mice (and the state of their bones became even better), so it is not clear how the therapy will work in the case of old and sick animals.
Cartilage tissue
More than 5 works on gene therapy are devoted to age-related pathologies of cartilage — degeneration of intervertebral discs and osteoarthritis — disorders associated with deformation and depletion of cartilage tissue, leading to chronic pain. It is important to note that all the work was carried out on adults, not old animals, in whom pathologies were caused surgically - damaging cartilage.
Several works were aimed at improving the structure of cartilage. For example, the Sox9 gene, which stimulates the production of the most important structural molecule, type 2 collagen, was delivered to the body. In other works, they suppressed inflammation in osteoarthritis. For example, siRNAs that suppress the activity of the pro-inflammatory NF-κB gene were delivered. All these approaches slowed the development of pathologies, but did not stop it.
However, one of the works deserves special attention! In this study, conducted by a Chinese scientist Wang, the longevity gene, SIRT6, was used. The introduction of this gene into the area of damaged cartilage slowed down both inflammation and cell aging, and the occurrence of osteoarthritis. It should be noted that in this work, the longevity mechanism common to most cells was affected. This gene can be used not only for the treatment of local age-related pathology, but also for the rejuvenation of the whole organism.
Muscle
The depletion of muscle tissue is one of the main causes of senile infirmity and decrepitude; and the fact that a person - starts to look like an old man. Sheer weakness and misery reaching shame. Exactly what is called life has twisted into a ram's horn. Therefore, its effective treatment is important not only for medicine, but also for cosmetology.
Today, in gene therapy of senile pathologies of muscles, only one approach is being developed, which, however, is very effective. This is the suppression of the activity of the myostatin gene, which interferes with muscle growth. Its “off” can be achieved, for example, by delivering the myostatin inhibitor gene, the peptide MyoPPT. According to Ketan Patel, its introduction to old mice leads to brilliant results: not only muscle mass increases significantly, but longevity genes also “turn on” (for example, FOXO).

Elizabeth Perrish
It is worth mentioning that Elizabeth Perrish, a woman who was the first in the world to undergo gene therapy of aging, introduced the myostatin inhibitor gene in addition to the telomerase gene to improve muscle function.
Immune system
Age - related changes affect the immune system - the body becomes more sensitive to viral and bacterial infections; mortality from them is increasing, and the prevention of diseases — vaccination — ceases to help. However, despite the importance of the problem, there are only 3 gene therapy works, and none of them can be considered quite successful. This is not surprising given the paucity of knowledge about the molecular and genetic mechanisms underlying the aging of the immune system.
Two papers are devoted to the treatment of age-related degeneration of the thymus, a central organ of the immune system in which T-lymphocytes mature (key participants in acquired immunity). In one of them, the gene for transcription factor FoxN1, regulating the development of the thymus and the formation of T cells there, was delivered to the thymus. And in the other, blood supply to the thymus was improved by delivery of the VEGF gene, vascular endothelial growth factor. However, in both cases, scientists managed to achieve only a slight slowdown in the degradation of the thymus.
And in the third work with the help of gene therapy they tried to improve the effectiveness of vaccination in old age. To do this, the IL-2 gene, the most important mediator of inflammation and immunity, was introduced into the body, which indeed slightly enhanced the systemic immune response to vaccination. However, it still remained significantly weaker compared with young mice.
To date, pharmacology boasts the greatest success in the field of rejuvenation of immunity. Farm giant Novartis even conducted clinical trials of a rapamycin analog (mTOR, an anti-aging pathway inhibitor), to improve the response to vaccination in the elderly! The fact is that the suppression of chronic inflammation is not only one of the ways to rejuvenate the immune system, but also slows down the aging of the whole organism.
central nervous system
Gene therapy for senile pathologies of the central nervous system (CNS) is an actively explored and rapidly developing area - more than 10 papers have already been dedicated to it! And they were carried out not only on old rats and mice, but even on primates - rhesus monkeys. This is not surprising, given that neurodegenerative diseases (Alzheimer's and Parkinson's) are among the top 10 leading causes of death in the world, and treatment of senile pathologies of the central nervous system is an effective prevention of these diseases.
Most of the work focused on the rejuvenation of the hippocampus - the ancient structure of the brain responsible for the formation of memory. All approaches “aim” at mechanisms that are very specific for nerve cells — neurons. Scientists are trying to improve the structure of neurons - by introducing the gene for the structural protein Homer1c. Or make more efficient signal transmission between nerve cells (using the PkcD gene involved in synapse). Note that the genes in the hippocampus are delivered by direct injection into this area of the brain. In most studies, therapy led to improved memory and spatial learning in old mice or rats, sometimes even to the level of young animals. Unfortunately, only the very short-term effects of such therapy were investigated, so their real effectiveness remains unknown.
The most promising work was Matthew J. During, dedicated not to therapy, but to the prevention of cognitive impairment. The gene of the transcription factor CREB (which is involved in the formation of long-term memory) was introduced into the hippocampus of young rats, and when these rats grew old, it turned out that their memory is in much better condition!
Interestingly, in parallel with gene therapy, in Los Angeles, the development of an artificial rat hippocampus is underway! Such a hippocampus is modeled as a set of neural networks and is intended for implantation into the brain instead of the damaged one. It has already been tested on live rats, which effectively restored their memory impairments, and project leader Theodore Berger (Theodore Berger) is enthusiastic and promises to create an artificial human hippocampus by 2025!
One work on gene therapy is devoted to the rejuvenation of another brain structure - the substantia nigra, or more precisely, its division Pars compacta. This part of the brain is primarily responsible for controlling movements, but another of its functions is the synthesis of a neurotransmitter (a substance that provides signal transmission from one neuron to another) of dopamine.
Dopamine is part of the brain's "reward system"; it is produced during a positive experience - sex, eating tasty food or drugs - and causes pleasure. However, it is no less important for switching attention from one stage of cognitive activity to another, and its insufficiency leads to an increased inertia of thinking.
To rejuvenate the Pars compacta of the black substance activated the path of longevity and stress resistance - increased the stability of proteins in the cell! Adult rats were injected with the chaperone gene GRP78, which provides for the reposition of misfolded proteins or proteins after degradation. Then, they monitored the state of animals until old age, and found that gene therapy protected neurons from death, even if they were acted on by damaging substances (alpha synuclein).
And finally, 3 very interesting works were carried out, where gene therapy was used for systemic rejuvenation of the whole brain! For systemic delivery, genetic constructs were injected into the cerebrospinal fluid washing the various parts of the brain.
In one study, in the brain, systemically suppressed inflammation by inhibiting pro-inflammatory substances, cytokines IL-12 and IL-23 using miRNA, which rather successfully improved the cognitive abilities of mice. True, the experiments were carried out on animals with accelerated aging. In another work, the brain of very old rats systemically rejuvenated the genome of insulin-like growth factor-1 (IGF-1)! As is known, IGF-1 launches one of the main ways of aging in the body, but in the central nervous system its role is completely different - it is a neuroprotector. Systemic delivery of the IGF-1 gene to the brain significantly improved the motor activity of rats, but not to the level of the young.
And in a remarkable study by Mark H. Tuszynski, brain therapy of old rhesus monkeys was carried out with the NGF genome, a nerve growth factor, which is also a neuroprotector. The introduction of this gene was very effective - the number and size of nerve cells (cholinergic neurons) became as in young monkeys! In addition, the effect of therapy remained for a very long time - longer than a year.
It should be noted that the NGF gene has a very good reputation - for more than 15 years it has been successfully studied in human clinical trials for Alzheimer's disease.
All of these approaches show how a variety of ways can be achieved to improve brain activity in old age. Apparently, the use of combined approaches to gene therapy of the brain can even lead to its rejuvenation.
Reproductive system
A lot of work is devoted to gene therapy of sexual disorders in old age. In men, this is erectile dysfunction, which, according to the Massachusetts Male Aging Study, affects 39% of men aged 40 years and 67% - at the age of 70 years. And for women, the cessation of the reproductive period is menopause, which occurs on average at 50 years of age and leads to infertility. All work on gene therapy was carried out on old animals - rats or mice.
About 6 approaches to the treatment of erectile dysfunction have been developed, but all of them are very similar - they affect the specific mechanisms of the smooth muscles of the penis. In several works, they activated the signal molecule NO, necessary for muscle relaxation (eNOS, ecSOD, miRNA against arginase genes), and in others they introduced the genes of ion channels necessary for muscle functioning (CATF, Maxi-K). The most effective was, apparently, the delivery of the KATF gene - the ATP-dependent potassium channels necessary for normal muscle tone. In this case - rejuvenation reached the level of young animals.
Apparently, in any case, treatment of erectile dysfunction will be more effective if it is accompanied by the normalization of hormonal balance.
For gene therapy of the female reproductive system, only one work was undertaken, but it is very interesting! It affected the main hormonal regulator, the hypothalamus, and the IGF-1 gene, insulin-like growth factor, was introduced there. This deserves special attention, because, as we have said, at the level of the whole organism, IGF-1 triggers aging, but is a neuroprotector in the CNS. IGF-1 is also involved in the regulation of sex hormones (in particular, it controls the release of gonadotropin-releasing hormone (GnRH) in response to estrogens).
The IGF-1 gene was administered to middle-aged female rats in the hypothalamus, and after a few months, their estrus cycle was improved, and their ovaries remained in much better condition. Perhaps the improvements were indeed associated with an increase in the production of IGF-1 and its regulation of sex hormones. But it is possible that such therapy led to the rejuvenation of the hypothalamus and, as a result, rejuvenation of the organism and its systems!
In general, the developed approaches are quite interesting, but they are understudied. First of all, it concerns the gene therapy of the male reproductive system, where the long-term effects of the therapy, as well as possible side effects, have not been studied.
Age obesity
Another age-related pathology is an increase in body weight, and more precisely in fat volume. Obesity, on the one hand, greatly worsens the state of health (for example, increases the risk of diabetes), and on the other - makes the person ugly. So gene therapy of this pathology opens up new perspectives both for medicine and for cosmetology.
Works on gene therapy of obesity in old age conducted only 3, and they are all very interesting. In 2 studies for weight loss, they acted on the regulatory centers of the brain.
In one of the works, the gene of the neurotrophic factor GDNF, contributing to the survival of neurons, was introduced into the field of the black substance. As a result, old fatty mice have lost much weight, which is partly due to the fact that mice have eaten less, and partly due to the fact that gene therapy has led to the activation of hypothalamic neurons secreting corticotropin (CRH).
In another paper, the Pomc gene, proopiomelanocortin, the precursor of melanocortins, was introduced into the hypothalamus, which are important for the regulation of energy balance and glucose metabolism. The experiments were carried out on old fatty rats, which after treatment became very thin. Interestingly, at the beginning their appetite decreased dramatically, but recovered after a month, which did not prevent further weight loss. Weight loss was partly due to the fact that therapy increased the energy expenditure in adipose tissue — it dissipated in the form of heat. It is also very important that a number of health parameters improved in rats: the balance of lipids and glucose was normalized, and insulin sensitivity increased.
And, finally, in one of the works, muscle obesity was treated with systemic delivery of the Wnt gene, which controls early embryonic development, and in adult tissues — necessary for maintaining stem cells and regulating their differentiation (transformation into specialized cells). The Wnt gene was administered to adult rats in the muscles and monitored their health for a long time. Interestingly, the therapy unexpectedly led to very good results: the rats not only lost weight, although they ate very fatty foods, but also reduced the risk of age-related pathologies. They had normal glucose metabolism, triglyceride levels in the blood decreased, and, interestingly, the bone mineral density even increased!
In general, I would like to note that approaches to gene therapy of obesity are very promising - in addition to losing weight, markers of systemic rejuvenation were observed: normalization of glucose and fat metabolism, and even improvement in bone tissue! Apparently, this is due to the fact that in all of them they tried to treat obesity with systemic effects - rejuvenating the nervous system, normalizing hormonal balance or acting on the paths of stem cells.
Vision
Methods of gene therapy are developed for visual impairment in old age, in particular, age-related macular degeneration (age-related macular degeneration - AMD), one of the most frequent causes of vision loss after 65. However, it should be noted that AMD is considered not only an age-related pathology, but also officially recognized as a disease. It is associated with excessive growth of blood vessels and the death of visual cells - retinal photoreceptors.
All treatments for AMD using gene therapy are aimed at suppressing the formation of blood vessels - angiogenesis. To do this, as a rule, inhibit the work of the main stimulator of angiogenesis - VEGF - vascular endothelial growth factor.
For example, a portion of the VEGF receptor gene, sFlt1, which suppresses VEGF activity is delivered to cells. This approach is better developed than others: not only studies on mice have been conducted, but also the first stages of clinical trials on people with AMD have been launched.
The study, published in 2015, involved 8 patients of both sexes from 71 to 86 years old. The sFlt01.19 gene was injected into the eye area, and after 52 weeks, visual acuity improved in all patients. No side effects were observed in this study, which opens up prospects for further clinical trials and the introduction of medication into clinical practice.
Another approach to AMD gene therapy is also aimed at suppressing vascular growth, but using other genes, angiostatin and endostatin. It was also effective in testing both rabbits and macaques.
Thus, AMD gene therapy is very effective - and already at the stage of translation to the clinic. However, as a target, it uses a rather specific pathway of angiogenesis and inflammation - VEGF, which can hardly be of interest for systemic therapy of aging. Interestingly, it is used for treatment and other age-related pathologies, for example, disorders of the cardiovascular system. However, in their case - VEGF, on the contrary, must be activated!
In general, gene therapy for age-related pathologies is a fairly developed area compared to gene therapy for aging, however, it is incomparably less popular than gene therapy for all sorts of diseases. Recall that if about 1,415 clinical trials were launched on cancer gene therapy, only 40–45 studies were performed on gene therapy of age-related pathologies and then on mice, rats and monkeys. (True, clinical trials are conducted on patients with AMD, however this is because AMD is officially recognized as a disease). Such a “neglect” of gene therapy for age-related pathologies seems to be related to the general focus of medicine, not on the prevention of diseases, but on the treatment of diseases of existing ones.
With regard to work on gene therapy of age-related pathologies, it can be noted that there are promising, high-quality works, but they constitute no more than a quarter of all the studies performed. In most works, gene therapy leads to a fairly moderate improvement in health (and, as a rule, does not reach the level of young animals), the duration of the therapeutic effect is not studied, side effects are not studied.
There are, however, very promising, outstanding quality of work. In particular, many such works have been carried out on the gene therapy of brain disorders. This, for example, gene therapy of the brain of old rhesus monkeys with the NGF genome — a nerve growth factor that restored the number of nerve cells (cholinergic neurons) to the level of young animals! Many promising studies have been carried out on gene therapy for age-related obesity. They not only led to weight loss, but also improved a whole set of health indicators.
In general, gene therapy of age-related pathologies seems to me to be an effective way of preventing diseases and fighting senility. Apparently, it is particularly promising to combine it with the gene therapy of aging, as, for example, Elizabeth Perrish did by introducing the telomerase gene along with the myostatin inhibitor gene to rejuvenate the muscles.

Conclusion
In conclusion, it can be noted that although gene therapy of aging is still a very young field of science, however, it is actively developing in many different directions and now inspires great hopes.
All studies are still at the preclinical stage of testing (on model animals or in vitro cells), but gene therapy of aging is already catching up with such powerful and life-prolonging effects as nutrition restriction and geroprotectors (for example, rapamycin or metformin).
Looking at all the variety of targets and approaches of gene therapy, I want to compare them and try to identify the most promising areas of development.
Firstly, it is much more effective to carry out gene therapy of aging than to target individual age-related pathologies. Indeed, in all works on gene therapy of aging, there is not only an extension of life, but also a slowdown in the development of a number of age-related pathologies. Thus, in Maria Blasco's telomerase gene therapy, the life of old mice not only increased by 20%, but also increased insulin sensitivity, improved neuromuscular coordination, decreased risk of osteoporosis and the content of molecular markers of aging.
And Donsheng Kai, using the hypothalamus therapy with the NF-kb genome, has not only prolonged the life of mice by 10%, but also improved mental performance and muscle work. In addition, the structure of the muscles, skin and bones of such mice was also in a younger state, compared with control animals (without therapy).
And therapy of a separate age-related pathology reduces only the risk of a certain group of diseases, but cannot prevent death from any other age-related diseases.
The second, noteworthy point: among the approaches to gene therapy of aging, we can distinguish those that affect most cells of the body and require systemic delivery, and those that focus on aging-regulating structures (for example, the hypothalamus). The latter are much easier and more convenient to use, and at the moment they seem to be easier to implement.
The fact is that systemic exposure is very difficult to achieve, even with the current state of viral delivery systems. It is required that the vector introduced into the blood falls into a significant part of the cells throughout the body. And, although Maria Blasko succeeded brilliantly, there is evidence that it is not easy for other laboratories to repeat her success.
And in the case of hypothalamus therapy, it is necessary that the gene only gets into a small area of brain tissue. And this can be easily achieved by injecting a vector with a gene into the appropriate region.
Third, in order to achieve more substantial results in gene therapy of aging, it is necessary to combine different approaches. There is a lot of evidence that the impact on several paths of longevity at once gives a greater effect on life expectancy than the impact on them separately. For example, if you turn off the growth hormone gene in mice, they live 50% longer. And if such mice are also limited in nutrition, the effect will increase to 80%. And an extraordinary survivor - a naked digger - a rodent that lives 10 times longer than a mouse, differs from it by activating a whole set of longevity pathways: this is increased resistance to stress, protection against cancer, stability of protein molecules, protection against neurodegeneration, etc.
Thus, in order to develop a gene therapy that drastically prolongs life, it is necessary to act simultaneously on many paths of longevity. That is to combine the approaches of gene therapy, which are now being developed to increase the stability of the genome, the hypothalamus rejuvenation destruction senessentnyh cells, increase resistance to stress, improve mitochondrial maintenance of stem cell niches and so on.
If now the therapy of a single gene does not lead to an increase in life expectancy more than 20%, we hope that in the future, due to the complex therapy of aging, it will be possible to achieve truly fantastic results.
Author Anastasia Shubina
Literature
1. http://www.wiley.com//legacy/wileychi/genmed/clinical/
2. Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016 Jan 1; 351 (6268): 84–8. doi: 10.1126 / science.aad5227. Epub 2015 Dec 1.
3. Anderson WF, Blaese RM, Culver K. The Haze Ther. - 1990. - v.1 (3). - P.331-362.
4. Edelstein ML, Abedi MR, Wixon J., Edelstein RM, The Generation of Trials worldwide 1989–2004 - an overview // J Gene Med. - 2004. - v.6. - P.597-602.
5. Kay MA State-of-the-art gene-based therapies: the road ahead // Nat Rev Genet. - 2011. - v.12 (5) - p. 316-28.
6. Greider, CW & Blackburn, EH (1985) "Identification of Tetrahymena extracts of specific transferase activity in Tetrahymen extracts." Cell v.43, (2 Pt. 1) pp. 405-413.
7. Egan CA1, Savre-Train I, Shay JW, Wilson SE, Bourne WM. Analysis of telomere lengths in human corneal endothelial cells from donors of different ages. Invest Ophthalmol Vis Sci. 1998 Mar; 39 (3): 648–53.
8. Kang MK, Guo W, Park NH. Reproductive senescence of normal human oral keratinocytes is associated with the loss of telomerase activity. Cell Growth Differ. 1998 Jan; 9 (1): 85–95.
9. Ilmonen P, Panda B, Janina S, Dustin PJ, Michaela Th. Genetics of telomere length in wild derived house mice. Genetics of telomere length in wild derived house mice. August 21–23, 2014, Santa Clara, USA
10. Bodnar AG, Ouellette M., Frolkis M., Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S., Wright . - 1998. - v.279 (5349). - P.349–52.
11. Jiang XR., Jimenez G., Chang E., Frolkis M., Kusler B., Sage M., Beeche M., Bodnar A., Wahl G., Tlsty T., Chiu CP. It doesn’t induce changes associated with a transformed phenotype // Nat. Genet. - 1999. - v. 21. - P. 111–114.
12. Chung SA, Wei AQ, Connor DE, Webb GC, Molloy T., Pajic M., Diwan AD Nucleus pulposus cellular longevity by telomerase gene therapy // Spine. - 2007. - v.32 (11). - P.1188–96.
13. Bernardes de Jesus, B., Vera, E., Schneeberger, K., Tejera, AM, Ayuso, E., Bosch, F., Blasco, M.E.M. . - 2012. - .v.4 (8). - P.691-704.
14. Tomás-Loba A., Flores I., Fernández-Marcos PJ, Cayuela ML, Maraver A., Tejera A., Borrás C, Matheu A., Klatt P., Flores JM, Viña J., Serrano M., Blasco MA Telomerase reverse transcriptase aging in cancer-resistant mice // Cell. - 2008. - v. 135 (4). - P.609–22.
15. Bär C, Bernardes de Jesus B, Serrano R, Tejera A, Ayuso E, Jimenez V, Formentini I, Bobadilla M, Mizrahi J, de Martino A, Gomez G, Pisano D, Mulero F, Wollert KC, Bosch F, Blasco MA. Telomerase expression confers cardiooprotection in the adult mouse heart's acute myocardial infarction. Nat Commun. 2014 Dec 18; 5: 5863. doi: 10.1038 / ncomms6863.
16. Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming IKK-β, NF-κB and GnRH. Nature. 2013. v.497 (7448). P.211–6.
17. Michalkiewicz M, Knestaut KM, Bytchkova EY, Michalkiewicz T (2003) Transgenic rats Hypotension and reduced catecholamines in neuropeptide Y. Hypertension 41 (5): 1056-1062.
18. Aveleira CA, Botelho M, Carmo-Silva S, Pascoal JF, Ferreira-Marques M, Nóbrega C, Cortes L, Valero J, Sousa-Ferreira L, Álvaro AR, Santana M, Kügler S, Pereira de Almeida L, Cavadas C. Neuropeptide Y stimulates autophagy in hypothalamic neurons Proc Natl Acad Sci US A. 2015 Mar 31; 112 (13): E1642–51. doi: 10.1073 / pnas.1416609112. Epub 2015 Mar 16.
19. Baranowska B, et al. (2006) Neuroendocrine control of metabolic homeostasis in Polish centenarians. J Physiol Pharmacol 57 (Suppl 6): 55–61.
20. Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, De Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006. v314 (5800). P825–8.
21. Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, Lago CU, Zhang S, DuBois W, Ward T, deCabo R, Gavrilova O, Mock B, Finkel T. Increased mammalian lifespan and segmental growth of mTOR expression. Cell Rep. 2013 Sep 12; 4 (5): 913–20. doi: 10.1016 / j.celrep.2013.07.030. Epub 2013 Aug 29.
22. Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007 Jul 27; 130 (2): 247–58.
23. Yamashita et.al. (2012). SUBT1: Transforming of the human transgenital reverse transcriptase gene. Biochemical and Biophysical Research Communications. 417: 630–634.
24. Yuan et.al. (2012). SIRT1 is required for long-term growth of human mesenchymal stem cells. J Mol Med. 90: 389–400
25. Herranz et al. (2010) “Sirt1 for healthy aging and protects from metabolic syndrome-associated cancer.” Nat Commun 1: 3
26. Satoh et al. (2013) "Cellotab 1 in the DMH and LH." Cell Metab. 18 (3): 416–430
27. Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, Muñoz-Martin M, Gómez-López G, Cañamero M, Mulero F, Pastor J, Martinez S, Romanos E, Mar Gonzalez-Barroso M, Rial E, Valverde AM, Bischoff JR, Serrano M. Pten positively regulates energy expenditure, and longevity. Cell Metab. 2012 Mar 7; 15 (3): 382–94. doi: 10.1016 / j.cmet.2012.02.02.001.
28. Wu et.al. (2015). Overexpression of Sirtuin 6 suppresses cellular senescence and NF-κB mediated inflammatory responses in osteoarthritis development. Sci Rep. 5: 17602.
29. Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010 Feb; 30 (3): 871–84. doi: 10.1128 / MCB.01145–09. Epub 2009 Nov 23.
30. Li et al. (2005). Stabilization of Nrf2 by tBHQ Confers Protection of Neural Stem Cells. Toxicol. Sci. 83 (2): 313–328.
31. Kanninen et.al. (2009). Alzheimer's disease. Proc Natl Acad Sci US A. 106 (38): 16505–16510.
32. Wang et.al. (2014). CD36 upregulation mediated by intranasal LV-NRF2 treatment mitigates hypoxia-induced progression of Alzheimer's-like pathogenesis. Antioxid Redox Signal. 21 (16): 2208-2230.
33. Minagawa et al. (2011). Accelerated epithelial cell senescence in IPF and the inhibitory role of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 300 (3): L391-401.
34. Takasaka et.al. (2014) .Autophagy induction by SIRT6 through the regulation of human bronchial epithelial cell senescence. J Immunol. 192 (3): 958–968.
35. Liu et al. (2014). Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed Res Int. 2014: 902842.
36. Mao et al. (2012). Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc Natl Acad Sci USA. 109 (29): 11800–5.
37. Xu et.al. (2015). SIRT6 is in a PARP1-dependent manner. Cell cycle. 14 (2): 269-276.
38. Chung et.al. (2015). Molecular Insights into SIRT1 Protection Against UV BInduced Skin Fibroblast Senescence by Acetylation. J Gerontol A Biol Sci Med Sci. 70 (8): 959–968.
39. Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006. v314 (5800). P825–8.
40. Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012 Feb 22; 483 (7388): 218–21. doi: 10.1038 / nature10815.
41. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013; 75: 685–705. doi: 10.1146 / annurev-physiol-030212–183653. Epub 2012 Nov 8.
42. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays aging-associated disorders. Nature. 2011 Nov 2; 479 (7372): 232–6. doi: 10.1038 / nature10600.
43. Rera et al. (2011) “Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog.” Cell Metab. 14 (5): 623–634.
44. Zhao et al. (2013). The effect of overexpression of the PGC-1a on the mtDNA4834 common deletion in a cochlear marginal cell senescence model. Hearing Research. 296: 13–24
45. Jiang XY, Lu DB, Jiang YZ, Zhou LN, Cheng LQ, Chen B. PGC-1α helps to reduce the number of animals. Diabetes Res Clin Pract. 2013 Jun; 100 (3): 368–75. doi: 10.1016 / j.diabres.2013.03.036. Epub 2013 Apr 22.
46. Liu XP, Gao H, Huang XY, Chen YF, Feng XJ, He YH, Li ZM, Liu PQ. Peroxisome proliferator-activated receptor gamma coactivator 1 alpha protects cardiomyocytes from hypertrophy by suppressing the cells of the c4 signaling pathway. Transl Res. 2015 Nov; 166 (5): 459–473.e3. doi: 10.1016 / j.trsl.2015.06.003. Epub 2015 Jun 9.
47. Hollinger K, Selsby JT. PGC-1α gene transfer muscle muscle in dystrophic muscle following prolonged disease progress. Exp Physiol. 2015 Oct; 100 (10): 1145–58. doi: 10.1113 / EP085339. Epub 2015 Sep 8.
48. De Luca G, Ventura I, Sanghez V, Russo MT, Ajmone-Cat MA, Cacci E, Martire A, Popoli P, Falcone G, Michelini F, Crescenzi M, Degan P, Minghetti L, Bignami M, Calamandrei G. Prolonged lifespan with enhanced oxidative behavior in mice overexpressing the oxidized nucleoside triphosphatase hMTH1. Aging Cell. 2013 Aug; 12 (4): 695–705. doi: 10.1111 / acel.12094. Epub 2013 May 30.
49. Wang J, Geesman GJ, Hostikka SL, Atallah M, Blackwell B, Lee E, Cook PJ, Pasaniuc B, Shariat G, Halperin E, Dobke M, Rosenfeld MG, Jordan IK, Lunyak VV. Inhibit of activated pericentromeric SINE / Alu repeat self-renewal. Cell cycle. 2011 Sep 1; 10 (17): 3016–30. Epub 2011 Sep 1.
50. SNEV overexpression extends the life span of human endothelial cells. Voglauer R, Chang MW, Dampier B, Wieser M, Baumann K, Sterovsky T, Schreiber M, Katinger H, Grillari J. Exp Cell Res. 2006 Apr 1; 312 (6): 746–59. Epub 2006 Jan 4.
51. Wood J, Nan Jiang, Brian C Jones, Stephen L Helfand Maintaining repressive heterochromatin extends lifespan in drosophila. Abstracts of papers presented at the 2014 meeting on Molecular genetics of aging, September 29 - October 3, 2014, Cold Spring Harbor, USA.
52. Sidler et al. (2014). It is a role for the H3K9 trimethylation of human diploid lung fibroblasts. Aging 6 (7): 545–563.
53. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005 Sep 16; 309 (5742): 1829–33. Epub 2005 Aug 25.
54. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R , Nabeshima YI. Mutation of the mouse resembling aging. Nature. 1997 Nov 6; 390 (6655): 45–51.
55. Wang Y, Kuro-o M, Sun Z. Klotho and Ramping Smooth muscle cells through the cAMP-PKA pathway. Aging Cell. 2012 Jun; 11 (3): 410–7. doi: 10.1111 / j.1474–9726.2012.00796.x. Epub 2012 Feb 22.
56. Wang et.al. (2009). Klotho Gene Delivery Prevents the Progression of Spontaneous Hypertension and Renal Damage. Hypertension. 54 (4): 810.
57. Zhou et.al. (2015). Klotho Ameliorates Kidney Injury and Fibrosis and Normalizes Blood Pressure by Targeting the Renin-Angiotensin System. Am J Pathol. 185 (12): 3211–3223.
58. Deng M, Luo Y, Li Y, Yang Q, Deng X, Wu P, Ma. H. Mol Med Rep. 2015 Jul; 12 (1): 45–54. doi: 10.3892 / mmr.2015.3367. Epub 2015 Feb 17.
59. Shu L, Wu M, Wu M, Huang C, Xu. Pak J Pharm Sci. 2014 Nov; 27 (6 Suppl): 2095–9.
60. Dena B. Dubal et al., Life Extension Factor, Klotho Enhances Cognition, Cell Reports, 2014, DOI: 10.1016 / j.celrep.2014.03.076
61. Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo k multiple . Biochem Biophys Res Commun. 2000 Sep 24; 276 (2): 767–72.
62. Brown KA, Chu, Y., Lund DD, Heistad DD, Faraci FM, Amt Physiol Heart Circ Physiol. - 2006. - v.290 (6). - H2600-5.
63. Schmidt U., del Monte F., Miyamoto MI, Matsui T., Gwathmey JK, Rosenzweig A., Hajjar RJ hearts through di 2 2 (2 +) - ATPase / / Circulation. - 2000. - v.101 (7). - P. 790–6.
64. Schmidt, U., Zhu, X., Lebeche, D., Huq, F., Guerrero, JL, Hajjar, RJ In vivo, Cardiovasc Res. - 2005. - v.66 (2). - P.318–23.
65. Wang, H., Keiser, JA, Olszewski, B., Rosebury, W., Robertson, A., Kovesdi, I., Gordon, D. Delayed angiogenesis in VEGF // Int. Mol. Med. - 2004. - v.13 (4). - P.581-7.
66. https://www.heart.org/idc/groups/ahamah-public/@wcm/@sop/@smd/documents/downloadable/ucm_470704.pdf
67. Renault et.al. (2013). Hedgehog-Dependent Regulation of Angiogenesis and Myogenesis Is Impaired in Aged Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 33: 2858–2866
68. Tonne et.al. (2014). Cardiac BNP gene delivery prolongs survival in spontaneously hypertensive rats with overt hypertensive heart disease. Aging (Albany NY). 6 (4): 311–319.
69. Wang QL., Han QL., Kang J., Gou S.-H., Wang L.-Zh. Polyethylenimine-mediated BMP-7 gene transfection promotes the elderly rats // Academic Journal of Second Military Medical University. - 2008. - v.28 (5). - P.514-518.
70. Yue B., Lu B., Dai KR, Zhang XL, Yu CF, Lou JR, Tang TT, BMP2 Calcif Tissue Int. - 2005. - v.77 (6). - P.395–403.
71. Liu B, Tang J, Ji J, Gu J. Prevention of osteoporosis in parathyroid hormone. Biotechnol Lett. 2003 Dec; 25 (24): 2117–22.
72. Xie H, Xie PL, Luo XH, Wu XP, Zhou HD, Tang SY, Liao EY. Omentin-1 exerts bone-sparing effect in ovariectomized mice. Osteoporos Int. 2012 Apr; 23 (4): 1425–36. doi: 10.1007 / s00198–011–1697–8. Epub 2011 Jul 14.
73. Chen W, Baylink DJ, Brier-Jones J, Neises A, Kiroyan JB, Rundle CH, Lau KH, Zhang XB. PDGFB-based stem cell therapy therapy. Proc Natl Acad Sci US A. 2015 Jul 21; 112 (29): E3893–900. doi: 10.1073 / pnas.1501759112. Epub 2015 Jul 6.
74. Kostenuik et.al. (2004). Gene therapy with human recombinant osteoprotegerin reverses established osteopenia in ovariectomized mice. Bone 34: 656–664.
75. Yin et.al. (2015). Adenoviral vector-mediated overexpression of osteoprotegerin accelerates osteointegration of titanium implants in ovariectomized rats. Gene Ther. 22 (8): 636–644.
76. Paul et.al. (2003). Potential Use of Sox9 Gene Therapy for Intervertebral Degenerative Disc Disease. Spine (Phila Pa 1976). 28 (8): 755–763.
77. Chen et al. (2008). Supplement of adenoviral vector-mediated NF-kBp65-specific siRNA. Osteoarthritis and Cartilage. 16: 174–184
78. Hsieh et.al. (2010). Intraarticular Gene Transfer of Thrombospondin-1 Supports the Disease Progression of Experimental Osteoarthritis. J Orthop Res. 28 (10): 1300-1306.
79. Chu et al. (2013). Protective effect of lentivirus-mediated siRNA targeting ADAMTS-5 on cartilage degradation. INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 31: 1222–1228.
80. Pi et.al. (2015). Intra-articular delivery of anti-hif-2α siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Therapy. 22 (6): 439–448.
81. Wu et.al. (2015). Overexpression of Sirtuin 6 suppresses cellular senescence and NF-κB mediated inflammatory responses in osteoarthritis development. Sci Rep. 5: 17602.
82. Collins-Hooper et.al. (2014). Propeptide-mediated inhibition of myostatin increases muscle mass through inhibitory proteolytic pathways in aged mice. J Gerontol A Biol Sci Med Sci. 69 (9): 1049-1059.
83. Sun et al. (2010). Declining expression of a single epithelial cell-autonomous gene accelerates age-related thymic involution. Aging Cell. 9 (3): 347–357.
84. Chung et.al. (2014). Engineering the human thymic microenvironment to support thymopoiesis in vivo. Stem Cells. 32 (9): 2386–2396.
85. Fayad R., Zhang H., Quinn D., Huang Y., Qiao L. Oral administration with papillomavirus pseudovirus encoding IL-2 fully restores mucosal and systemic responses to vaccine in aged mice // J Immunol. - 2004. - v.173 (4). –P.2692–8.
86. Gerstein, H., Lindstrom, MJ, Burger C. Generation of cognitive aging. Neurobiol Aging. - 2013. - v.34 (8). - P.1963-70.
87. Zhang GR, Liu M., Cao H., Kong L., Wang X., O'Brien JA, Wu SC, Cook RG, Geller AI. of hippocampal neurons // Hippocampus. - 2009. - v.19 (5). - P.413–23.
88. Sun M., Kong L., Wang X., Holmes C., Gao Q., Zhang W., Pfeilschifter J., Goldstein DS, Geller AI Coexpression of tyrosine hydroxylase, GTP cyclohydrolase I, aromatic amino acid decarboxylase, and vesicular monoamine transporter-2 from aV-1 vector supports high-level, model for Parkinson's disease // Hum Gene Ther. - 2004. - v.15. –P.1177–1196.
89. Mouravlev A., Dunning, J., Young D., During the decaying of the rats, for example, Proc Natl Acad Sci US A. - 2006. - v.103 (12 ). - P.4705–10.
90. Pertusa M., García-Matas S., Mammeri H., Adell A., Rodrigo T., Mallet J., Cristòfol R., Sarkis C., Sanfeliu C. Expression of GDNF transgene in astrocytes rats // Neurobiol Aging. - 2008. - v.29 (9). - P.1366–79.
91. Nishida F., Morel GR, Hereñú CB, Schwerdt JI, Goya RG, Portiansky EL Restorative effect of the intracerebroventricular insulin-like growth factor / IG, Neuroscience. - 2011. - v.177. - P.195–206.
92. Nagahara AH, Bernot T., Moseanko R., Brignolo L., Blesch A., Conner JM, Ramirez A., Gasmi M., Tuszynski MH Long-term reversal of cholinergic neuronal decline by age NGF gene delivery // Exp Neurol. - 2009. - v.215 (1). - P.153–9.
93. Rodríguez SS, Schwerdt JI, Barbeito CG, Flamini MA, Han Y., Bohn MC, Goya RG Hypothalamic IGF-I and IGF-I. Endocrinology. - 2013. - v.154 (6). - P.2166–73.
94. Bivalacqua TJ, Armstrong JS, Biggerstaff J., Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ, Champion HC. Ami Physiol Heart Circ Physiol. - 2003. - v.284 (4). - H1408-21.
95. Bivalacqua TJ, Deng W., Kendirci M., Usta MF, Robinson C., Taylor BK, Murthy SN, Champion HC, Hellstrom WJG, Kadowitz PJ Mesenchymal stem cells -associated erectile dysfunction // Am J Physiol Heart Circ Physiol. - 2007. - v.292. - H1278 – H1290.
96. Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HCN. Am J Physiol Heart Circ Physiol. - 2007. - v.292 (3). - H1340–51. a.
97. Bivalacqua TJ, Champion HC, Mehta YS, Abdel-Mageed AB, Sikka SC, Ignarro LJ, Kadowitz PJ, Hellstrom WJ AHDT (eNOS) to the penis, agerelated erectile dysfunction the rat Int J Impot Res. - 2000. - Suppl 3: S8 – S17.
98. So I., Chae MR, Lee. SW Gene Transformer (ATP) channel restores age-related erectile dysfunction in rats // BJU Int. - 2007. - v.100 (5). - P.1154-60.
99. Melman, A., Biggs, G., Davies, K., Zhao, W., Tar. MT, Christ. . - 2008. - v.15 (5). - P.364-70.
100. Tümer N., Scarpace PJ, Dogan MD, Broxson CS, Matheny M., Yurek DM, Peden CS, Burger C., Muzyczka N., Mandel RJ Hypothalamic rAAV-mediated GDNF. Aging. - 2006. - v.27 (3). - P.459-70.
101. Boghossian S., Ueno N., Dube MG, Kalra P., Kalra S. Leptin leptin // Neurobiol Aging. - 2007. - v.28 (10). - P.1594-604.
102. Aslanidi G1, Kroutov V, Philipsberg G, Lamb K, Campbell-Thompson M, Walter GA, Kurenov S, Ignacio Aguirre J, Keller P, Hankenson K, Macdougald OA, Zolotukhin S. Ectopic expression homeostasis in obese rats. Am J Physiol Endocrinol Metab. 2007 Sep; 293 (3): E726–36. Epub 2007 Jun 19.
103. Manfredsson FP1, Tumer N, Erdos B, Landa T, Broxson CS, Sullivan LF, Rising AC, Foust KD, Zhang Y, Muzyczka N, Gorbatyuk OS, Scarpace PJ, Mandel RJ. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss. Mol Ther. 2009 Jun; 17 (6): 980–91. doi: 10.1038 / mt. Epub 2009 Mar 10.
104. MacLachlan et.al. (2011). Preclinical Safety Evaluation of AAV2-sFLT01 - A Gene Therapy for Age-Related Macular Degeneration. The American Society of Gene & Cell Therapy. 19 (2): 326–334
105. Binley et al. (2012). Equine Infectious Anemia Virus-Based Gene Therapy, RetinoStat, for Age-Related Macular Degeneration. HUMAN GENE THERAPY. 23: 980–991
106. Rakoczy et.al. (2015). Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomized clinical trial. Lancet.
107. Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. drosophila dFOXO controls lifespan and regulates insulin signaling in the brain. Nature. 2004 Jun 3; 429 (6991): 562-6.
Liu et al. (2015). Senescence of human skin-derived precursors regulated by Akt-FOXO3-p27KIP1 / p15INK4b signaling. Cell. M
2. Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016 Jan 1; 351 (6268): 84–8. doi: 10.1126 / science.aad5227. Epub 2015 Dec 1.
3. Anderson WF, Blaese RM, Culver K. The Haze Ther. - 1990. - v.1 (3). - P.331-362.
4. Edelstein ML, Abedi MR, Wixon J., Edelstein RM, The Generation of Trials worldwide 1989–2004 - an overview // J Gene Med. - 2004. - v.6. - P.597-602.
5. Kay MA State-of-the-art gene-based therapies: the road ahead // Nat Rev Genet. - 2011. - v.12 (5) - p. 316-28.
6. Greider, CW & Blackburn, EH (1985) "Identification of Tetrahymena extracts of specific transferase activity in Tetrahymen extracts." Cell v.43, (2 Pt. 1) pp. 405-413.
7. Egan CA1, Savre-Train I, Shay JW, Wilson SE, Bourne WM. Analysis of telomere lengths in human corneal endothelial cells from donors of different ages. Invest Ophthalmol Vis Sci. 1998 Mar; 39 (3): 648–53.
8. Kang MK, Guo W, Park NH. Reproductive senescence of normal human oral keratinocytes is associated with the loss of telomerase activity. Cell Growth Differ. 1998 Jan; 9 (1): 85–95.
9. Ilmonen P, Panda B, Janina S, Dustin PJ, Michaela Th. Genetics of telomere length in wild derived house mice. Genetics of telomere length in wild derived house mice. August 21–23, 2014, Santa Clara, USA
10. Bodnar AG, Ouellette M., Frolkis M., Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S., Wright . - 1998. - v.279 (5349). - P.349–52.
11. Jiang XR., Jimenez G., Chang E., Frolkis M., Kusler B., Sage M., Beeche M., Bodnar A., Wahl G., Tlsty T., Chiu CP. It doesn’t induce changes associated with a transformed phenotype // Nat. Genet. - 1999. - v. 21. - P. 111–114.
12. Chung SA, Wei AQ, Connor DE, Webb GC, Molloy T., Pajic M., Diwan AD Nucleus pulposus cellular longevity by telomerase gene therapy // Spine. - 2007. - v.32 (11). - P.1188–96.
13. Bernardes de Jesus, B., Vera, E., Schneeberger, K., Tejera, AM, Ayuso, E., Bosch, F., Blasco, M.E.M. . - 2012. - .v.4 (8). - P.691-704.
14. Tomás-Loba A., Flores I., Fernández-Marcos PJ, Cayuela ML, Maraver A., Tejera A., Borrás C, Matheu A., Klatt P., Flores JM, Viña J., Serrano M., Blasco MA Telomerase reverse transcriptase aging in cancer-resistant mice // Cell. - 2008. - v. 135 (4). - P.609–22.
15. Bär C, Bernardes de Jesus B, Serrano R, Tejera A, Ayuso E, Jimenez V, Formentini I, Bobadilla M, Mizrahi J, de Martino A, Gomez G, Pisano D, Mulero F, Wollert KC, Bosch F, Blasco MA. Telomerase expression confers cardiooprotection in the adult mouse heart's acute myocardial infarction. Nat Commun. 2014 Dec 18; 5: 5863. doi: 10.1038 / ncomms6863.
16. Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming IKK-β, NF-κB and GnRH. Nature. 2013. v.497 (7448). P.211–6.
17. Michalkiewicz M, Knestaut KM, Bytchkova EY, Michalkiewicz T (2003) Transgenic rats Hypotension and reduced catecholamines in neuropeptide Y. Hypertension 41 (5): 1056-1062.
18. Aveleira CA, Botelho M, Carmo-Silva S, Pascoal JF, Ferreira-Marques M, Nóbrega C, Cortes L, Valero J, Sousa-Ferreira L, Álvaro AR, Santana M, Kügler S, Pereira de Almeida L, Cavadas C. Neuropeptide Y stimulates autophagy in hypothalamic neurons Proc Natl Acad Sci US A. 2015 Mar 31; 112 (13): E1642–51. doi: 10.1073 / pnas.1416609112. Epub 2015 Mar 16.
19. Baranowska B, et al. (2006) Neuroendocrine control of metabolic homeostasis in Polish centenarians. J Physiol Pharmacol 57 (Suppl 6): 55–61.
20. Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, De Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006. v314 (5800). P825–8.
21. Wu JJ, Liu J, Chen EB, Wang JJ, Cao L, Narayan N, Fergusson MM, Rovira II, Allen M, Springer DA, Lago CU, Zhang S, DuBois W, Ward T, deCabo R, Gavrilova O, Mock B, Finkel T. Increased mammalian lifespan and segmental growth of mTOR expression. Cell Rep. 2013 Sep 12; 4 (5): 913–20. doi: 10.1016 / j.celrep.2013.07.030. Epub 2013 Aug 29.
22. Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007 Jul 27; 130 (2): 247–58.
23. Yamashita et.al. (2012). SUBT1: Transforming of the human transgenital reverse transcriptase gene. Biochemical and Biophysical Research Communications. 417: 630–634.
24. Yuan et.al. (2012). SIRT1 is required for long-term growth of human mesenchymal stem cells. J Mol Med. 90: 389–400
25. Herranz et al. (2010) “Sirt1 for healthy aging and protects from metabolic syndrome-associated cancer.” Nat Commun 1: 3
26. Satoh et al. (2013) "Cellotab 1 in the DMH and LH." Cell Metab. 18 (3): 416–430
27. Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, Muñoz-Martin M, Gómez-López G, Cañamero M, Mulero F, Pastor J, Martinez S, Romanos E, Mar Gonzalez-Barroso M, Rial E, Valverde AM, Bischoff JR, Serrano M. Pten positively regulates energy expenditure, and longevity. Cell Metab. 2012 Mar 7; 15 (3): 382–94. doi: 10.1016 / j.cmet.2012.02.02.001.
28. Wu et.al. (2015). Overexpression of Sirtuin 6 suppresses cellular senescence and NF-κB mediated inflammatory responses in osteoarthritis development. Sci Rep. 5: 17602.
29. Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010 Feb; 30 (3): 871–84. doi: 10.1128 / MCB.01145–09. Epub 2009 Nov 23.
30. Li et al. (2005). Stabilization of Nrf2 by tBHQ Confers Protection of Neural Stem Cells. Toxicol. Sci. 83 (2): 313–328.
31. Kanninen et.al. (2009). Alzheimer's disease. Proc Natl Acad Sci US A. 106 (38): 16505–16510.
32. Wang et.al. (2014). CD36 upregulation mediated by intranasal LV-NRF2 treatment mitigates hypoxia-induced progression of Alzheimer's-like pathogenesis. Antioxid Redox Signal. 21 (16): 2208-2230.
33. Minagawa et al. (2011). Accelerated epithelial cell senescence in IPF and the inhibitory role of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 300 (3): L391-401.
34. Takasaka et.al. (2014) .Autophagy induction by SIRT6 through the regulation of human bronchial epithelial cell senescence. J Immunol. 192 (3): 958–968.
35. Liu et al. (2014). Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed Res Int. 2014: 902842.
36. Mao et al. (2012). Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence. Proc Natl Acad Sci USA. 109 (29): 11800–5.
37. Xu et.al. (2015). SIRT6 is in a PARP1-dependent manner. Cell cycle. 14 (2): 269-276.
38. Chung et.al. (2015). Molecular Insights into SIRT1 Protection Against UV BInduced Skin Fibroblast Senescence by Acetylation. J Gerontol A Biol Sci Med Sci. 70 (8): 959–968.
39. Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006. v314 (5800). P825–8.
40. Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012 Feb 22; 483 (7388): 218–21. doi: 10.1038 / nature10815.
41. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013; 75: 685–705. doi: 10.1146 / annurev-physiol-030212–183653. Epub 2012 Nov 8.
42. Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays aging-associated disorders. Nature. 2011 Nov 2; 479 (7372): 232–6. doi: 10.1038 / nature10600.
43. Rera et al. (2011) “Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog.” Cell Metab. 14 (5): 623–634.
44. Zhao et al. (2013). The effect of overexpression of the PGC-1a on the mtDNA4834 common deletion in a cochlear marginal cell senescence model. Hearing Research. 296: 13–24
45. Jiang XY, Lu DB, Jiang YZ, Zhou LN, Cheng LQ, Chen B. PGC-1α helps to reduce the number of animals. Diabetes Res Clin Pract. 2013 Jun; 100 (3): 368–75. doi: 10.1016 / j.diabres.2013.03.036. Epub 2013 Apr 22.
46. Liu XP, Gao H, Huang XY, Chen YF, Feng XJ, He YH, Li ZM, Liu PQ. Peroxisome proliferator-activated receptor gamma coactivator 1 alpha protects cardiomyocytes from hypertrophy by suppressing the cells of the c4 signaling pathway. Transl Res. 2015 Nov; 166 (5): 459–473.e3. doi: 10.1016 / j.trsl.2015.06.003. Epub 2015 Jun 9.
47. Hollinger K, Selsby JT. PGC-1α gene transfer muscle muscle in dystrophic muscle following prolonged disease progress. Exp Physiol. 2015 Oct; 100 (10): 1145–58. doi: 10.1113 / EP085339. Epub 2015 Sep 8.
48. De Luca G, Ventura I, Sanghez V, Russo MT, Ajmone-Cat MA, Cacci E, Martire A, Popoli P, Falcone G, Michelini F, Crescenzi M, Degan P, Minghetti L, Bignami M, Calamandrei G. Prolonged lifespan with enhanced oxidative behavior in mice overexpressing the oxidized nucleoside triphosphatase hMTH1. Aging Cell. 2013 Aug; 12 (4): 695–705. doi: 10.1111 / acel.12094. Epub 2013 May 30.
49. Wang J, Geesman GJ, Hostikka SL, Atallah M, Blackwell B, Lee E, Cook PJ, Pasaniuc B, Shariat G, Halperin E, Dobke M, Rosenfeld MG, Jordan IK, Lunyak VV. Inhibit of activated pericentromeric SINE / Alu repeat self-renewal. Cell cycle. 2011 Sep 1; 10 (17): 3016–30. Epub 2011 Sep 1.
50. SNEV overexpression extends the life span of human endothelial cells. Voglauer R, Chang MW, Dampier B, Wieser M, Baumann K, Sterovsky T, Schreiber M, Katinger H, Grillari J. Exp Cell Res. 2006 Apr 1; 312 (6): 746–59. Epub 2006 Jan 4.
51. Wood J, Nan Jiang, Brian C Jones, Stephen L Helfand Maintaining repressive heterochromatin extends lifespan in drosophila. Abstracts of papers presented at the 2014 meeting on Molecular genetics of aging, September 29 - October 3, 2014, Cold Spring Harbor, USA.
52. Sidler et al. (2014). It is a role for the H3K9 trimethylation of human diploid lung fibroblasts. Aging 6 (7): 545–563.
53. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005 Sep 16; 309 (5742): 1829–33. Epub 2005 Aug 25.
54. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R , Nabeshima YI. Mutation of the mouse resembling aging. Nature. 1997 Nov 6; 390 (6655): 45–51.
55. Wang Y, Kuro-o M, Sun Z. Klotho and Ramping Smooth muscle cells through the cAMP-PKA pathway. Aging Cell. 2012 Jun; 11 (3): 410–7. doi: 10.1111 / j.1474–9726.2012.00796.x. Epub 2012 Feb 22.
56. Wang et.al. (2009). Klotho Gene Delivery Prevents the Progression of Spontaneous Hypertension and Renal Damage. Hypertension. 54 (4): 810.
57. Zhou et.al. (2015). Klotho Ameliorates Kidney Injury and Fibrosis and Normalizes Blood Pressure by Targeting the Renin-Angiotensin System. Am J Pathol. 185 (12): 3211–3223.
58. Deng M, Luo Y, Li Y, Yang Q, Deng X, Wu P, Ma. H. Mol Med Rep. 2015 Jul; 12 (1): 45–54. doi: 10.3892 / mmr.2015.3367. Epub 2015 Feb 17.
59. Shu L, Wu M, Wu M, Huang C, Xu. Pak J Pharm Sci. 2014 Nov; 27 (6 Suppl): 2095–9.
60. Dena B. Dubal et al., Life Extension Factor, Klotho Enhances Cognition, Cell Reports, 2014, DOI: 10.1016 / j.celrep.2014.03.076
61. Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo k multiple . Biochem Biophys Res Commun. 2000 Sep 24; 276 (2): 767–72.
62. Brown KA, Chu, Y., Lund DD, Heistad DD, Faraci FM, Amt Physiol Heart Circ Physiol. - 2006. - v.290 (6). - H2600-5.
63. Schmidt U., del Monte F., Miyamoto MI, Matsui T., Gwathmey JK, Rosenzweig A., Hajjar RJ hearts through di 2 2 (2 +) - ATPase / / Circulation. - 2000. - v.101 (7). - P. 790–6.
64. Schmidt, U., Zhu, X., Lebeche, D., Huq, F., Guerrero, JL, Hajjar, RJ In vivo, Cardiovasc Res. - 2005. - v.66 (2). - P.318–23.
65. Wang, H., Keiser, JA, Olszewski, B., Rosebury, W., Robertson, A., Kovesdi, I., Gordon, D. Delayed angiogenesis in VEGF // Int. Mol. Med. - 2004. - v.13 (4). - P.581-7.
66. https://www.heart.org/idc/groups/ahamah-public/@wcm/@sop/@smd/documents/downloadable/ucm_470704.pdf
67. Renault et.al. (2013). Hedgehog-Dependent Regulation of Angiogenesis and Myogenesis Is Impaired in Aged Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 33: 2858–2866
68. Tonne et.al. (2014). Cardiac BNP gene delivery prolongs survival in spontaneously hypertensive rats with overt hypertensive heart disease. Aging (Albany NY). 6 (4): 311–319.
69. Wang QL., Han QL., Kang J., Gou S.-H., Wang L.-Zh. Polyethylenimine-mediated BMP-7 gene transfection promotes the elderly rats // Academic Journal of Second Military Medical University. - 2008. - v.28 (5). - P.514-518.
70. Yue B., Lu B., Dai KR, Zhang XL, Yu CF, Lou JR, Tang TT, BMP2 Calcif Tissue Int. - 2005. - v.77 (6). - P.395–403.
71. Liu B, Tang J, Ji J, Gu J. Prevention of osteoporosis in parathyroid hormone. Biotechnol Lett. 2003 Dec; 25 (24): 2117–22.
72. Xie H, Xie PL, Luo XH, Wu XP, Zhou HD, Tang SY, Liao EY. Omentin-1 exerts bone-sparing effect in ovariectomized mice. Osteoporos Int. 2012 Apr; 23 (4): 1425–36. doi: 10.1007 / s00198–011–1697–8. Epub 2011 Jul 14.
73. Chen W, Baylink DJ, Brier-Jones J, Neises A, Kiroyan JB, Rundle CH, Lau KH, Zhang XB. PDGFB-based stem cell therapy therapy. Proc Natl Acad Sci US A. 2015 Jul 21; 112 (29): E3893–900. doi: 10.1073 / pnas.1501759112. Epub 2015 Jul 6.
74. Kostenuik et.al. (2004). Gene therapy with human recombinant osteoprotegerin reverses established osteopenia in ovariectomized mice. Bone 34: 656–664.
75. Yin et.al. (2015). Adenoviral vector-mediated overexpression of osteoprotegerin accelerates osteointegration of titanium implants in ovariectomized rats. Gene Ther. 22 (8): 636–644.
76. Paul et.al. (2003). Potential Use of Sox9 Gene Therapy for Intervertebral Degenerative Disc Disease. Spine (Phila Pa 1976). 28 (8): 755–763.
77. Chen et al. (2008). Supplement of adenoviral vector-mediated NF-kBp65-specific siRNA. Osteoarthritis and Cartilage. 16: 174–184
78. Hsieh et.al. (2010). Intraarticular Gene Transfer of Thrombospondin-1 Supports the Disease Progression of Experimental Osteoarthritis. J Orthop Res. 28 (10): 1300-1306.
79. Chu et al. (2013). Protective effect of lentivirus-mediated siRNA targeting ADAMTS-5 on cartilage degradation. INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 31: 1222–1228.
80. Pi et.al. (2015). Intra-articular delivery of anti-hif-2α siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Therapy. 22 (6): 439–448.
81. Wu et.al. (2015). Overexpression of Sirtuin 6 suppresses cellular senescence and NF-κB mediated inflammatory responses in osteoarthritis development. Sci Rep. 5: 17602.
82. Collins-Hooper et.al. (2014). Propeptide-mediated inhibition of myostatin increases muscle mass through inhibitory proteolytic pathways in aged mice. J Gerontol A Biol Sci Med Sci. 69 (9): 1049-1059.
83. Sun et al. (2010). Declining expression of a single epithelial cell-autonomous gene accelerates age-related thymic involution. Aging Cell. 9 (3): 347–357.
84. Chung et.al. (2014). Engineering the human thymic microenvironment to support thymopoiesis in vivo. Stem Cells. 32 (9): 2386–2396.
85. Fayad R., Zhang H., Quinn D., Huang Y., Qiao L. Oral administration with papillomavirus pseudovirus encoding IL-2 fully restores mucosal and systemic responses to vaccine in aged mice // J Immunol. - 2004. - v.173 (4). –P.2692–8.
86. Gerstein, H., Lindstrom, MJ, Burger C. Generation of cognitive aging. Neurobiol Aging. - 2013. - v.34 (8). - P.1963-70.
87. Zhang GR, Liu M., Cao H., Kong L., Wang X., O'Brien JA, Wu SC, Cook RG, Geller AI. of hippocampal neurons // Hippocampus. - 2009. - v.19 (5). - P.413–23.
88. Sun M., Kong L., Wang X., Holmes C., Gao Q., Zhang W., Pfeilschifter J., Goldstein DS, Geller AI Coexpression of tyrosine hydroxylase, GTP cyclohydrolase I, aromatic amino acid decarboxylase, and vesicular monoamine transporter-2 from aV-1 vector supports high-level, model for Parkinson's disease // Hum Gene Ther. - 2004. - v.15. –P.1177–1196.
89. Mouravlev A., Dunning, J., Young D., During the decaying of the rats, for example, Proc Natl Acad Sci US A. - 2006. - v.103 (12 ). - P.4705–10.
90. Pertusa M., García-Matas S., Mammeri H., Adell A., Rodrigo T., Mallet J., Cristòfol R., Sarkis C., Sanfeliu C. Expression of GDNF transgene in astrocytes rats // Neurobiol Aging. - 2008. - v.29 (9). - P.1366–79.
91. Nishida F., Morel GR, Hereñú CB, Schwerdt JI, Goya RG, Portiansky EL Restorative effect of the intracerebroventricular insulin-like growth factor / IG, Neuroscience. - 2011. - v.177. - P.195–206.
92. Nagahara AH, Bernot T., Moseanko R., Brignolo L., Blesch A., Conner JM, Ramirez A., Gasmi M., Tuszynski MH Long-term reversal of cholinergic neuronal decline by age NGF gene delivery // Exp Neurol. - 2009. - v.215 (1). - P.153–9.
93. Rodríguez SS, Schwerdt JI, Barbeito CG, Flamini MA, Han Y., Bohn MC, Goya RG Hypothalamic IGF-I and IGF-I. Endocrinology. - 2013. - v.154 (6). - P.2166–73.
94. Bivalacqua TJ, Armstrong JS, Biggerstaff J., Abdel-Mageed AB, Kadowitz PJ, Hellstrom WJ, Champion HC. Ami Physiol Heart Circ Physiol. - 2003. - v.284 (4). - H1408-21.
95. Bivalacqua TJ, Deng W., Kendirci M., Usta MF, Robinson C., Taylor BK, Murthy SN, Champion HC, Hellstrom WJG, Kadowitz PJ Mesenchymal stem cells -associated erectile dysfunction // Am J Physiol Heart Circ Physiol. - 2007. - v.292. - H1278 – H1290.
96. Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HCN. Am J Physiol Heart Circ Physiol. - 2007. - v.292 (3). - H1340–51. a.
97. Bivalacqua TJ, Champion HC, Mehta YS, Abdel-Mageed AB, Sikka SC, Ignarro LJ, Kadowitz PJ, Hellstrom WJ AHDT (eNOS) to the penis, agerelated erectile dysfunction the rat Int J Impot Res. - 2000. - Suppl 3: S8 – S17.
98. So I., Chae MR, Lee. SW Gene Transformer (ATP) channel restores age-related erectile dysfunction in rats // BJU Int. - 2007. - v.100 (5). - P.1154-60.
99. Melman, A., Biggs, G., Davies, K., Zhao, W., Tar. MT, Christ. . - 2008. - v.15 (5). - P.364-70.
100. Tümer N., Scarpace PJ, Dogan MD, Broxson CS, Matheny M., Yurek DM, Peden CS, Burger C., Muzyczka N., Mandel RJ Hypothalamic rAAV-mediated GDNF. Aging. - 2006. - v.27 (3). - P.459-70.
101. Boghossian S., Ueno N., Dube MG, Kalra P., Kalra S. Leptin leptin // Neurobiol Aging. - 2007. - v.28 (10). - P.1594-604.
102. Aslanidi G1, Kroutov V, Philipsberg G, Lamb K, Campbell-Thompson M, Walter GA, Kurenov S, Ignacio Aguirre J, Keller P, Hankenson K, Macdougald OA, Zolotukhin S. Ectopic expression homeostasis in obese rats. Am J Physiol Endocrinol Metab. 2007 Sep; 293 (3): E726–36. Epub 2007 Jun 19.
103. Manfredsson FP1, Tumer N, Erdos B, Landa T, Broxson CS, Sullivan LF, Rising AC, Foust KD, Zhang Y, Muzyczka N, Gorbatyuk OS, Scarpace PJ, Mandel RJ. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss. Mol Ther. 2009 Jun; 17 (6): 980–91. doi: 10.1038 / mt. Epub 2009 Mar 10.
104. MacLachlan et.al. (2011). Preclinical Safety Evaluation of AAV2-sFLT01 - A Gene Therapy for Age-Related Macular Degeneration. The American Society of Gene & Cell Therapy. 19 (2): 326–334
105. Binley et al. (2012). Equine Infectious Anemia Virus-Based Gene Therapy, RetinoStat, for Age-Related Macular Degeneration. HUMAN GENE THERAPY. 23: 980–991
106. Rakoczy et.al. (2015). Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomized clinical trial. Lancet.
107. Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. drosophila dFOXO controls lifespan and regulates insulin signaling in the brain. Nature. 2004 Jun 3; 429 (6991): 562-6.
Liu et al. (2015). Senescence of human skin-derived precursors regulated by Akt-FOXO3-p27KIP1 / p15INK4b signaling. Cell. M
All Articles