Guide to electrical materials for all. Part 1
Hi Giktayms! I decided to publish in parts my manual on materials used not only in electrical engineering, but also in general in engineering, including home-craftsmen. With a description, examples of use, notes on the work. The manual is written as simple as possible, and it will be clear to everyone, from the student to the pensioner.
In this part, we begin to disassemble the conductors - Silver, Copper, Aluminum.

Welcome to CAT (TRAFFIC)
Picking around in search of answers to my questions in various textbooks on materials science, textbooks, and scientific books, I was terrified that the academic style of presentation builds a wall between those who want to learn and knowledge. As far as the desire of the authors to circumvent the sharp edges, the dark places turns the textbooks into a uniform endless desert of boredom and despair. At the same time, the beyond-the-limit level of abstraction makes it extremely difficult for a neophyte to use the knowledge gained in practice. So I decided to make my guide, with blackjack and prodigal girls.
This manual is a living one, as new materials are received, clarified, and comments are received from you, dear readers, it will be supplemented, changed, and made better. I always have the freshest version of the manual on my website in Blozhik. I support the Open Source and Open Hardware movement with both hands. I believe that the exchange of knowledge should be free, it will be useful for everyone, therefore the manual is distributed under the license Creative Commons 3.0 BY-NC- SA, which means you can do anything with it: upload, distribute, modify, observing only three restrictions:
The benefits of this manual:
Publishing the guide here I really hope for an abundance of constructive criticism and additions from you, dear readers.
Go!
The twentieth century is the century of plastics. Before the advent of a wide range of synthetic polymeric materials, man used in the design of metals and materials of natural origin - wood, leather, etc. Today we are inundated with plastic products, ranging from disposable tableware, to heavily loaded parts of car engines. Plastics are in many ways superior to metals, but they will never displace them completely, so the story will begin with metals. There are hundreds of books devoted to metals, and the discipline dedicated to them is called “metallography”.
We are interested in metals in terms of electronic technology. As conductors, as part of electronic devices. All other applications, such as construction materials, are not included in this manual.
The main thing for electronic technology is the ability of metals to conduct electricity well. Let's look at the resistivity table of various metals:
We see the leaders of our list: Ag, Cu, Au, Al.
Ag - Silver. A precious metal. Silver is the cheapest of precious metals, but, nevertheless, too expensive to make wires from it. 5% better electrical conductivity compared to copper, with a price difference of almost 100 times.
In the form of coatings of conductors in the microwave technology. The high frequency current, due to the skin effect, flows over the surface of the conductor, and not in its thickness, so a thin coating of the waveguide with silver gives a greater increase in conductivity than the coating of silver with a conductor for direct current.
In contact group alloys. Contacts of power, signal relays, knife switches, switches are most often made of an alloy containing silver. The contact resistance of such a contact is obtained below copper, it is less susceptible to oxidation. Since the contact is usually miniaturized, the cost of this small silver additive to the value of the product is negligible. Although the disposal of a large number of relays, the cost of silver makes it advisable to work with side cutters to separate the contacts into a pile for subsequent refining.

The contacts of the power relay are 16 amps. According to the manufacturer's documentation
contacts contain silver and cadmium.

Various relays. The upper relay has even a silvered case with a characteristic patina. The content of precious metals in products manufactured in the USSR was indicated in the passports for the products.
As an additive in solders. Quality solders (both hard and soft) often contain silver.
Conductive coatings on dielectrics. For example, to obtain a contact pad on ceramics, a suspension of silver particles is applied to it, followed by roasting in a furnace (“burn-in” method).
Component of electrically conductive adhesives and paints. Conductive ink often
contain suspension of silver particles. As the ink dries, the solvent
evaporates, the particles in the solution are getting closer, sticking together and creating conductive
bridges through which current can flow. A good video recipe for creating such
ink.
Despite the fact that silver is a noble metal, it is oxidized in a medium containing
sulfur:
4Ag + 2H 2 S + O 2 → 2Ag 2 S + 2H 2 O
Formed a dark patina - "patina". Also a source of sulfur can serve as rubber,
this is a rubber-insulated wire and silver-plated contacts are a bad combination.
Darkened silver can be chemically purified. Unlike cleaning with abrasive pastes (including toothpaste), this is the most gentle way of cleaning that does not leave scratches.
Cu - copper. The main metal conductor current. The windings of electric motors, wires in insulation, tires, flexible conductors - most often it is copper. Copper is not difficult to recognize by its characteristic reddish color. Copper is sufficiently resistant to corrosion.
Wires. The main use of copper in its pure form. Any additives reduce the electrical conductivity, so the core of the wires is usually the purest copper.

Flexible multicore wires of various sections.
Flexible tokovody. If the conductors for stationary devices can, in principle, be made of any metal, then flexible conductors are almost always made only of copper, aluminum for these purposes is too brittle. They contain many thin copper streaks.
Heat sinks. Copper is not only 56% better than aluminum that conducts current, but also has almost twice the best thermal conductivity. Heat pipes, radiators, heat distribution plates are made of copper. Since copper is more expensive than aluminum, often radiators are made of composite, the core of copper, and the rest of the cheaper aluminum.

CPU cooling radiators. The central core is made of copper, it removes well the heat from the processor chip, and the aluminum heatsink with developed fins already cools the core itself.
In the manufacture of foil printed circuit boards. Printed circuit boards, in any electronic device, are made of a dielectric plate on which copper foil is glued. All connections between the elements of the printed circuit board are made of copper foil.
Technique of ultra high vacuum. Of metals and alloys, only stainless steel and copper are suitable for ultra-high vacuum chambers in such devices as particle accelerators or X-ray spectrometers. All other metals in a vacuum evaporate slightly and spoil the vacuum.
Anodes of x-ray tubes. In X-ray analysis, monochromatic X-rays are required. Its source is often copper-irradiated by electrons (Cu Kα spectral line), which also removes heat perfectly. If another radiation is required (Co or Fe), it is obtained from a small piece of the corresponding metal on a massive copper heat sink. Such anodes are always cooled with running water.
Al - Aluminum. "Winged metal" is the fourth conduction after silver, gold and copper.
Although aluminum conducts a current almost one and a half times worse than copper, it is 3.4 times lighter and three times lighter.
times cheaper. And if you count the conductivity, then the equivalent copper conductor from
Aluminum will be cheaper by 6.5 times! Aluminum would displace copper as a conductor everywhere if
would not be a pair of its nasty properties, but this is in shortcomings.
Pure aluminum, like pure iron, is practically not used in technology (exceptions
- wires and foil). Any "aluminum" object consists of some kind of aluminum alloy. Alloys may contain silicon, magnesium, copper, zinc and other metals. Their properties are very different, and this must be taken into account during processing. The following are some of the most common grades of aluminum:
The relatively low melting point (660 ° C for pure, less than 600 ° C for casting alloys) aluminum makes it possible to cast parts into sand molds under conditions
garage / workshop. However, many aluminum grades are not suitable for casting.
Wires. Aluminum is cheap, so it is advantageous to make thick power cables, CIP , power lines from aluminum. In old houses, apartment wiring was made with aluminum wire (since 2001 PUE prohibits the use of aluminum wire in apartments, only copper, see PUE 7, edition 7.1.34). Also, aluminum is not used as a conductor in demanding applications.

Left old aluminum wire. On the right are aluminum cables of various sections,
suitable for laying in the ground. In particular, the cable on the right was connected to electricity the whole floor of the building. The cable in addition to the outer rubber sheath has armor steel tape, to protect the underlying insulation from damage, for example with a shovel during excavation.
Heat sinks. Not only home batteries are made of aluminum, but also radiators
microchips, processors, made from aluminum.
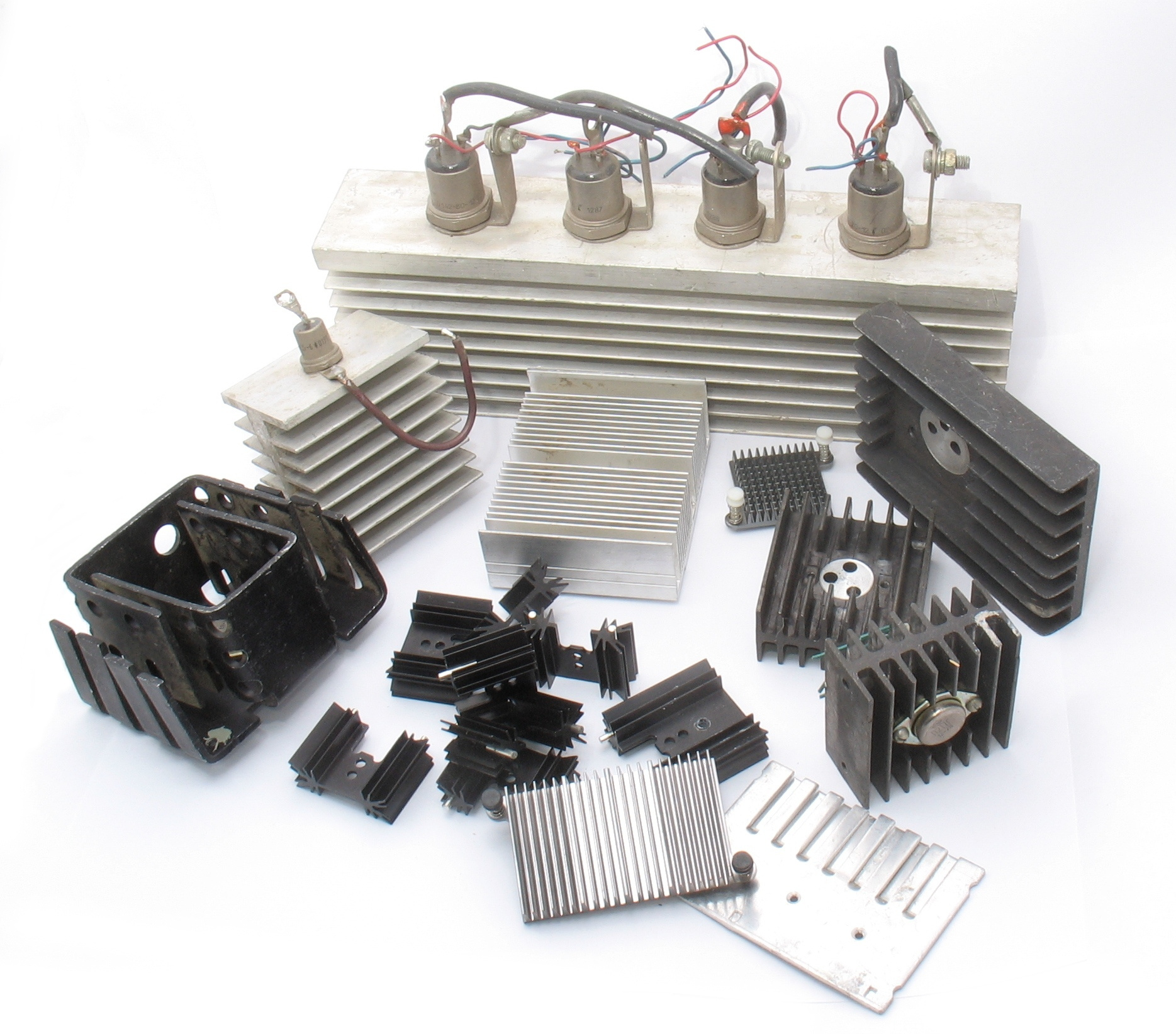
Various aluminum radiators.
Instrument cases. The hard disk case in your computer is molded from aluminum alloy. A small addition of silicon improves the strength properties of aluminum, the alloy silumin is the case of hard drives, household appliances, gearboxes, etc.
Anodized aluminum (aluminum, whose electrochemical oxide film
on the surface it is made thicker and stronger) it is well painted and just beautiful. Oxide
the film (Al 2 O 3 - gems rubies and sapphires consist of the same substance) is quite hard and wear-resistant, but unfortunately the aluminum under it is soft, and with a strong impact it breaks like ice on water.
Screens Electromagnetic shielding is often made from aluminum foil or thin aluminum tin. You can do a simple mobile phone experiment.
wrapped in foil will lose the net - it will be shielded.
Reflective coating at the mirrors. A thin film of aluminum on glass reflects 89% of the incident light (approximate value, depending on conditions) (Silver is 98%, but darkens in air due to sulfur compounds). Any laser printer contains a rotating mirror, covered with a thin layer of aluminum.
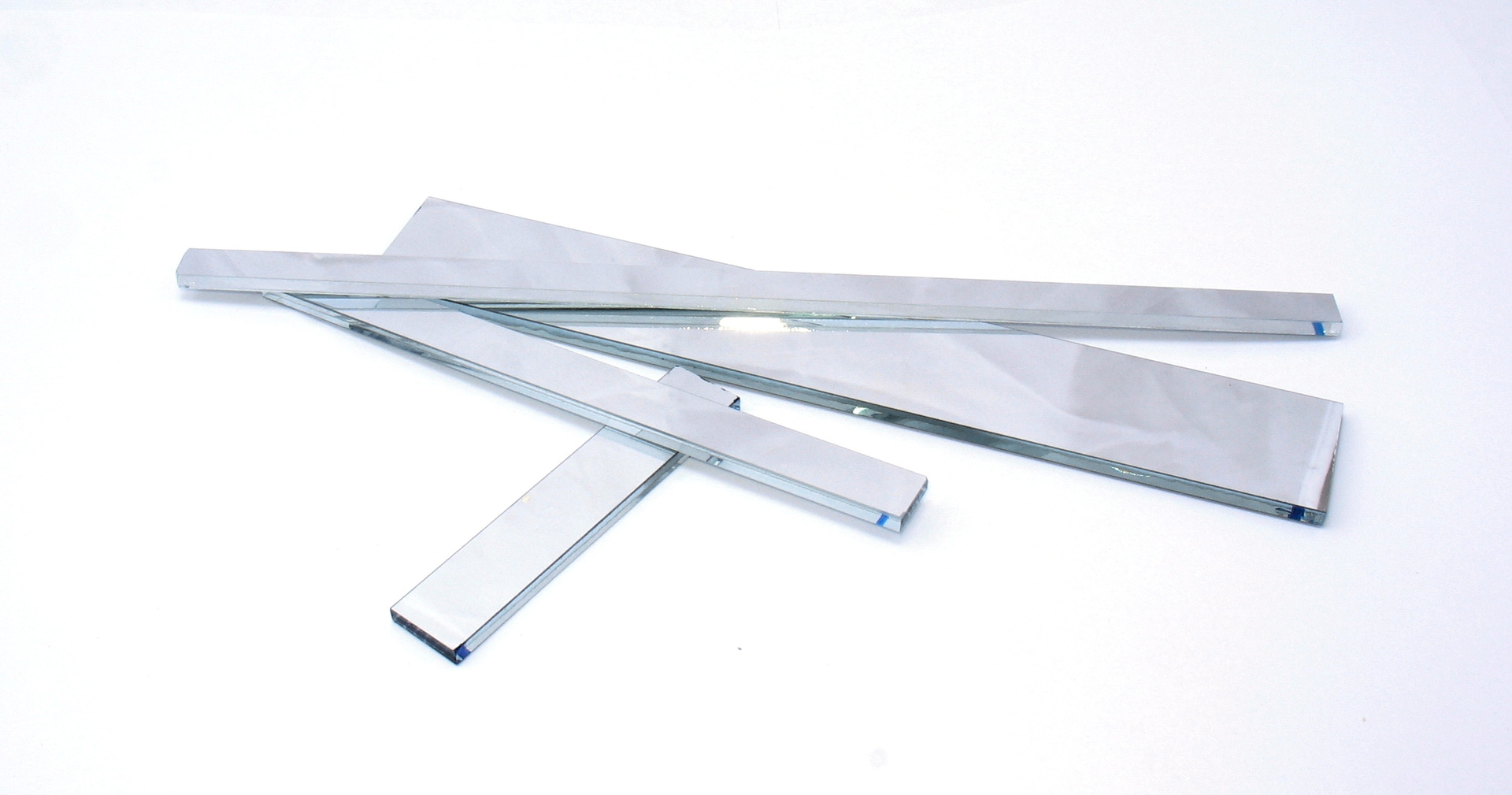
Mirrors from the optical system of the flatbed scanner. Please note that optical mirrors have metallized glass on the outside, in contrast to the usual household mirrors, where there is a reflective coating to protect behind the glass. Household mirrors give a double reflection - from the surface of the glass and from the reflective coating, which is not so critical in everyday life as the security of the reflective coating.
Electrodes of capacitor plates. Aluminum foil, separated by a layer of dielectric and tightly rolled into a cylinder, is part of the electric capacitor (however, to reduce the size of the capacitor, the foil is replaced with an aluminum coating). The fact that the aluminum oxide film is thin, durable and does not conduct current is used in electrolytic capacitors, which have enormous electrical capacitances for their dimensions.
Aluminum is an active metal , but in air it is covered with an oxide film, which protects the metal from destruction and hides its active nature. If aluminum is not allowed to form a stable protective film, for example, with a drop of mercury, aluminum actively reacts with water. Aluminum dissolves in an alkaline environment, try pouring aluminum foil with a pipe cleaning agent - the reaction will be violent, with release of explosive hydrogen. The chemical activity of aluminum, paired with a large difference in electronegativity with copper, makes it impossible to directly connect wires of these two metals. In the presence of moisture (there is almost always air in the air), galvanic corrosion begins to occur with the destruction of aluminum.

Two identical transformers from microwave ovens. The left one failed due to aluminum windings - the wire was burned out from the contact - aluminum is poorly soldered with soft solders, an attempt to ensure contact as well as that of the copper wire led to breakage.
Aluminum creeping. If the aluminum wire is very tight, it is deformed.
and retain a new shape - this is called "plastic deformation." If not squeeze it
so much so that it does not deform, but leave it under load for a long time - aluminum
will begin to "crawl" changing shape gradually. This dirty property leads to what is good
tightened terminal with aluminum wire after 5-10-20 years will gradually weaken and will
hang out without providing the former electrical contact. This is one of the reasons why the PUE
Prohibits thin aluminum wire for wiring electricity by consumers in
buildings. In industry, it is not difficult to ensure the regulation - the so-called "broach"
panel, when an electrician periodically checks the tightness of all terminals in the panel. At home, however, usually while the socket with the smoke does not burn - no one will attend to the quality of the contact. A bad contact is the cause of the fires.
Aluminum, in comparison with copper, is less ductile , the risk of a knife on a conductor, when removing insulation from a wire will quickly lead to a broken conductor than copper, therefore insulation from aluminum wires should be brushed off like a pencil, at an angle, and not at the end.
Once again, an important note. Aluminum and copper conductors cannot be directly connected! To connect conductors of copper and aluminum, use an intermediate metal, for example, a steel terminal.
In large building stores (OBI, Leroy Merlin, Castorama), aluminum profiles of various sizes and shapes are usually available. A good source can be extruded aluminum dishes - it is very cheap and there are different forms. But pay attention to the brand. If you need 6061 and even more so 7075, you will have to buy it from a company specializing in metals.
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final
In this part, we begin to disassemble the conductors - Silver, Copper, Aluminum.

Welcome to CAT (TRAFFIC)
An introduction that nobody normally reads.
Picking around in search of answers to my questions in various textbooks on materials science, textbooks, and scientific books, I was terrified that the academic style of presentation builds a wall between those who want to learn and knowledge. As far as the desire of the authors to circumvent the sharp edges, the dark places turns the textbooks into a uniform endless desert of boredom and despair. At the same time, the beyond-the-limit level of abstraction makes it extremely difficult for a neophyte to use the knowledge gained in practice. So I decided to make my guide, with blackjack and prodigal girls.
This manual is a living one, as new materials are received, clarified, and comments are received from you, dear readers, it will be supplemented, changed, and made better. I always have the freshest version of the manual on my website in Blozhik. I support the Open Source and Open Hardware movement with both hands. I believe that the exchange of knowledge should be free, it will be useful for everyone, therefore the manual is distributed under the license Creative Commons 3.0 BY-NC- SA, which means you can do anything with it: upload, distribute, modify, observing only three restrictions:
- The link to me is obligatory (incl. Derivative works).
- You cannot earn on my allowance without an agreement with me (a ban on the use for commercial purposes).
- All derivative works must be distributed under the same conditions.
The benefits of this manual:
- The whole text was written by me, and supplemented by the wonderful people mentioned in the Thanks section. I did not include information in which I would doubt the veracity or relevance. Therefore, the proportion of bullshit in the text is on average lower than in marketing texts of resellers-suppliers, but higher than in a good Soviet textbook.
- I at least felt most of the materials, used them in my constructions, and did not see only in the picture.
- The manual is completely (To be completely honest - with the exception of one picture, which I had to draw in what I could do.) Prepared using OpenSource products (Linux, GIMP, LibreOffice, context). Just for fun.
- Some sections have the item "Sources" - tips on finding materials - where to buy, under what names to search. Of course, everything can be bought on Aliexpress and on Ebay, so this option is not indicated. The item can be useful if the material is needed "here and now."
Publishing the guide here I really hope for an abundance of constructive criticism and additions from you, dear readers.
Contents of the manual
Guides:
*Silver
*Copper
*Aluminum
*Iron
*Gold
*Nickel
*Tungsten
*Mercury
So-so conductors:
*Carbon
* Nichromes
* Alloys for the manufacture of thermostable resistance
* Solders
*Tin
* Fusible solders
Other conductors
* Thermocouple alloys
* India-Tin Oxide
Dielectrics (Not at all conductors):
* Inorganic dielectrics
**Porcelain
**Glass
**Mica
** Aluminum oxide ceramics
**Asbestos
**Water
* Semi-synthetic organic dielectrics
** Paper, cardboard
**Silk
** Wax, paraffin wax
** Transformer oil
** Plywood, chipboard
* Organic synthetic dielectrics
** Materials based on phenol-formaldehyde resins
** Carbolite (bakelite)
** Getinaks
** Textolite
** steklotekstolit
** Varnished
**Rubber
**Ebonite
**Polyethylene
**Polypropylene
** Polystyrene, ABS plastic
** Fluoroplast-4 (PTFE polytetrafluoroethylene)
** Polyvinyl chloride - PVC
** Polyethylene terephthalate (PET)
** Silicones
** Polyimide
** Polyamides
** Polymethylmethacrylate - PMMA
** Polycarbonate
* Graph of the history of industrial application of polymers
* Electrical tape
** Rubberized fabric electrical tape
** Fabric tape
** Rubber self vulcanizing electrical tape
** Silicone self-sticking tapes
** Polyimide tape
** PVC electrical tape
** Stationery adhesive tape
* Insulating tubes
** PVC tube - cambric
** Fluoroplastic tube
** Glass cloth with silicone
**Heat-shrink tubing
* Additional Polymer Information
*Silver
*Copper
*Aluminum
*Iron
*Gold
*Nickel
*Tungsten
*Mercury
So-so conductors:
*Carbon
* Nichromes
* Alloys for the manufacture of thermostable resistance
* Solders
*Tin
* Fusible solders
Other conductors
* Thermocouple alloys
* India-Tin Oxide
Dielectrics (Not at all conductors):
* Inorganic dielectrics
**Porcelain
**Glass
**Mica
** Aluminum oxide ceramics
**Asbestos
**Water
* Semi-synthetic organic dielectrics
** Paper, cardboard
**Silk
** Wax, paraffin wax
** Transformer oil
** Plywood, chipboard
* Organic synthetic dielectrics
** Materials based on phenol-formaldehyde resins
** Carbolite (bakelite)
** Getinaks
** Textolite
** steklotekstolit
** Varnished
**Rubber
**Ebonite
**Polyethylene
**Polypropylene
** Polystyrene, ABS plastic
** Fluoroplast-4 (PTFE polytetrafluoroethylene)
** Polyvinyl chloride - PVC
** Polyethylene terephthalate (PET)
** Silicones
** Polyimide
** Polyamides
** Polymethylmethacrylate - PMMA
** Polycarbonate
* Graph of the history of industrial application of polymers
* Electrical tape
** Rubberized fabric electrical tape
** Fabric tape
** Rubber self vulcanizing electrical tape
** Silicone self-sticking tapes
** Polyimide tape
** PVC electrical tape
** Stationery adhesive tape
* Insulating tubes
** PVC tube - cambric
** Fluoroplastic tube
** Glass cloth with silicone
**Heat-shrink tubing
* Additional Polymer Information
Go!
Guides
The twentieth century is the century of plastics. Before the advent of a wide range of synthetic polymeric materials, man used in the design of metals and materials of natural origin - wood, leather, etc. Today we are inundated with plastic products, ranging from disposable tableware, to heavily loaded parts of car engines. Plastics are in many ways superior to metals, but they will never displace them completely, so the story will begin with metals. There are hundreds of books devoted to metals, and the discipline dedicated to them is called “metallography”.
We are interested in metals in terms of electronic technology. As conductors, as part of electronic devices. All other applications, such as construction materials, are not included in this manual.
The main thing for electronic technology is the ability of metals to conduct electricity well. Let's look at the resistivity table of various metals:
| Metal | Resistivity Ohm * mm2 / m |
|---|---|
| Silver | 0,015 ... 0,0162 |
| Copper | 0.01724 ... 0.018 |
| Gold | 0.023 |
| Aluminum | 0.0262 ... 0.0295 |
| Iridium | 0.0474 |
| Tungsten | 0.053 ... 0.055 |
| Molybdenum | 0.054 |
| Zinc | 0.059 |
| Nickel | 0.087 |
| Iron | 0.098 |
| Platinum | 0.107 |
| Tin | 0.12 |
| Lead | 0,217 ... 0,227 |
| Titanium | 0.5562 ... 0.7837 |
| Bismuth | 1.2 |
We see the leaders of our list: Ag, Cu, Au, Al.
Silver
Ag - Silver. A precious metal. Silver is the cheapest of precious metals, but, nevertheless, too expensive to make wires from it. 5% better electrical conductivity compared to copper, with a price difference of almost 100 times.
Application examples
In the form of coatings of conductors in the microwave technology. The high frequency current, due to the skin effect, flows over the surface of the conductor, and not in its thickness, so a thin coating of the waveguide with silver gives a greater increase in conductivity than the coating of silver with a conductor for direct current.
In contact group alloys. Contacts of power, signal relays, knife switches, switches are most often made of an alloy containing silver. The contact resistance of such a contact is obtained below copper, it is less susceptible to oxidation. Since the contact is usually miniaturized, the cost of this small silver additive to the value of the product is negligible. Although the disposal of a large number of relays, the cost of silver makes it advisable to work with side cutters to separate the contacts into a pile for subsequent refining.

The contacts of the power relay are 16 amps. According to the manufacturer's documentation
contacts contain silver and cadmium.

Various relays. The upper relay has even a silvered case with a characteristic patina. The content of precious metals in products manufactured in the USSR was indicated in the passports for the products.
As an additive in solders. Quality solders (both hard and soft) often contain silver.
Conductive coatings on dielectrics. For example, to obtain a contact pad on ceramics, a suspension of silver particles is applied to it, followed by roasting in a furnace (“burn-in” method).
Component of electrically conductive adhesives and paints. Conductive ink often
contain suspension of silver particles. As the ink dries, the solvent
evaporates, the particles in the solution are getting closer, sticking together and creating conductive
bridges through which current can flow. A good video recipe for creating such
ink.
disadvantages
Despite the fact that silver is a noble metal, it is oxidized in a medium containing
sulfur:
4Ag + 2H 2 S + O 2 → 2Ag 2 S + 2H 2 O
Formed a dark patina - "patina". Also a source of sulfur can serve as rubber,
this is a rubber-insulated wire and silver-plated contacts are a bad combination.
Darkened silver can be chemically purified. Unlike cleaning with abrasive pastes (including toothpaste), this is the most gentle way of cleaning that does not leave scratches.
Copper
Cu - copper. The main metal conductor current. The windings of electric motors, wires in insulation, tires, flexible conductors - most often it is copper. Copper is not difficult to recognize by its characteristic reddish color. Copper is sufficiently resistant to corrosion.
Application examples
Wires. The main use of copper in its pure form. Any additives reduce the electrical conductivity, so the core of the wires is usually the purest copper.

Flexible multicore wires of various sections.
Flexible tokovody. If the conductors for stationary devices can, in principle, be made of any metal, then flexible conductors are almost always made only of copper, aluminum for these purposes is too brittle. They contain many thin copper streaks.
Heat sinks. Copper is not only 56% better than aluminum that conducts current, but also has almost twice the best thermal conductivity. Heat pipes, radiators, heat distribution plates are made of copper. Since copper is more expensive than aluminum, often radiators are made of composite, the core of copper, and the rest of the cheaper aluminum.

CPU cooling radiators. The central core is made of copper, it removes well the heat from the processor chip, and the aluminum heatsink with developed fins already cools the core itself.
In the manufacture of foil printed circuit boards. Printed circuit boards, in any electronic device, are made of a dielectric plate on which copper foil is glued. All connections between the elements of the printed circuit board are made of copper foil.
Technique of ultra high vacuum. Of metals and alloys, only stainless steel and copper are suitable for ultra-high vacuum chambers in such devices as particle accelerators or X-ray spectrometers. All other metals in a vacuum evaporate slightly and spoil the vacuum.
Anodes of x-ray tubes. In X-ray analysis, monochromatic X-rays are required. Its source is often copper-irradiated by electrons (Cu Kα spectral line), which also removes heat perfectly. If another radiation is required (Co or Fe), it is obtained from a small piece of the corresponding metal on a massive copper heat sink. Such anodes are always cooled with running water.
Interesting facts about copper
- Copper is a rather expensive metal, so unscrupulous manufacturers try to save on it. The cross section of the wires is underestimated (when written 0.75 mm2, and in fact 0.11 mm2). Aluminum is painted “under the copper” in the windings, the outside winding looks like copper, and if you scrape off the insulation, it turns out that it is made of aluminum. This is the fault of both Chinese and domestic manufacturers, the cable with a section of 2.5 mm2 may well be a section of 2.3 mm2, so the safety factor and input control will not be superfluous. Of course, the reliability of the contact in the electrical fittings of the core with a cross section of 2.3 mm2, designed for a core of 2.5 mm2, will be low.
- Copper colors the flame green, this property was used to detect copper in the ore when chemical analysis was not available. Green footprint in the flame - an indicator of the presence of copper. (but not always, boron ions can give a green flame)
- Copper is a soft metal, but if you add at least 10% tin to copper, you get a hard, elastic alloy — bronze. It was the mastering of the production of bronze that served as the name for the historical epoch - the bronze age. An additive to beryllium copper gives beryllium bronze - a durable elastic alloy from which spring contacts are made.
- Copper is one of the few soft metals with a high melting point; therefore, copper seals are made of copper, for example, for high-temperature or vacuum technology. For example, the sealing gasket plugs crankcase car engine.
- During machining (for example, drawing), copper is compacted and becomes rigid. To restore the original softness and plasticity, copper is “annealed” in a protective atmosphere, heating to 500–700 ° C and keeping it for some time. Therefore, some copper products are hard, and some soft, such as copper pipes.
- Copper does not spark. For work in explosive places, for example on a gas pipeline, an intrinsically safe tool, a steel tool coated with a copper layer or a tool made of copper alloys - bronze are used. If such a tool accidentally strike on the steel surface, it will not give dangerous sparks.
- Since the temperature coefficient of resistance for pure copper is known, resistance thermometers are made of copper (type TCM - Copper Resistance Thermometer, there is also TSP - Platinum Resistance Thermometer). A resistance thermometer is a precision-made resistor, wound from copper wire. Having measured its resistance, it is possible to determine its temperature quite accurately using a table or a formula.
Aluminum
Al - Aluminum. "Winged metal" is the fourth conduction after silver, gold and copper.
Although aluminum conducts a current almost one and a half times worse than copper, it is 3.4 times lighter and three times lighter.
times cheaper. And if you count the conductivity, then the equivalent copper conductor from
Aluminum will be cheaper by 6.5 times! Aluminum would displace copper as a conductor everywhere if
would not be a pair of its nasty properties, but this is in shortcomings.
Pure aluminum, like pure iron, is practically not used in technology (exceptions
- wires and foil). Any "aluminum" object consists of some kind of aluminum alloy. Alloys may contain silicon, magnesium, copper, zinc and other metals. Their properties are very different, and this must be taken into account during processing. The following are some of the most common grades of aluminum:
- 1199. Pure 99.99% aluminum. It happens almost exclusively in the form of foil.
- 1050 and 1060. Pure 99.5% and 99.6%, respectively. Due to its high thermal conductivity it is sometimes used as a material for radiators. Soft, easy to bend. Wires, food foil, dishes.
- 6061 and 6082. Alloys: 6061 - Si 0.6%, Mg 1.0%, Cu 0.28%, 6082 - Si, Mg, Mn. The first is more common in the United States, the second in Europe. Easy to sharpen, mill. The best material for homemade. Other Easy to weld, soldered. Easy to anodize. Bad bends. Not suitable for casting.
- 6060. Composition: Mg, Si. Softer than 6061 and 6082, when machining, slightly “clay”, for which turners do not like it. Distributed and cheap, has no other special advantages. Cheap aluminum profile of an incomprehensible alloy has good chances to be them.
- 5083. Alloy with magnesium (> 4% Mg). Excellent corrosion resistance, resistant to seawater. One of the best options for parts working in the rain. It can also be found in a building materials store, along with other similar brands.
- 44400, he is "silumin". An alloy with a high percentage of silicon (Si> 8%). Casting. Low melting point, when brazing solder the risk of melting the part itself. Fragile, bends when broken. On a break visible characteristic crystals.
- 7075. 2.1-2.9% Mg, 5.1-6.1% Zn, 1.2-1.6% Cu. Very peculiar alloy, it differs even in color (the film of oxides is slightly golden). Suddenly hard for aluminum, comparable in hardness to mild steel. Badly anodized. Not soldered at all. Does not weld at all. Do not bend and not forged at all. Not suitable for casting. It cuts perfectly, perfectly polished. Good for responsible parts. Used for screws in bicycles, in arms (the material of many parts of the rifle M16).
The relatively low melting point (660 ° C for pure, less than 600 ° C for casting alloys) aluminum makes it possible to cast parts into sand molds under conditions
garage / workshop. However, many aluminum grades are not suitable for casting.
Application examples
Wires. Aluminum is cheap, so it is advantageous to make thick power cables, CIP , power lines from aluminum. In old houses, apartment wiring was made with aluminum wire (since 2001 PUE prohibits the use of aluminum wire in apartments, only copper, see PUE 7, edition 7.1.34). Also, aluminum is not used as a conductor in demanding applications.

Left old aluminum wire. On the right are aluminum cables of various sections,
suitable for laying in the ground. In particular, the cable on the right was connected to electricity the whole floor of the building. The cable in addition to the outer rubber sheath has armor steel tape, to protect the underlying insulation from damage, for example with a shovel during excavation.
Heat sinks. Not only home batteries are made of aluminum, but also radiators
microchips, processors, made from aluminum.

Various aluminum radiators.
Instrument cases. The hard disk case in your computer is molded from aluminum alloy. A small addition of silicon improves the strength properties of aluminum, the alloy silumin is the case of hard drives, household appliances, gearboxes, etc.
Anodized aluminum (aluminum, whose electrochemical oxide film
on the surface it is made thicker and stronger) it is well painted and just beautiful. Oxide
the film (Al 2 O 3 - gems rubies and sapphires consist of the same substance) is quite hard and wear-resistant, but unfortunately the aluminum under it is soft, and with a strong impact it breaks like ice on water.
Screens Electromagnetic shielding is often made from aluminum foil or thin aluminum tin. You can do a simple mobile phone experiment.
wrapped in foil will lose the net - it will be shielded.
Reflective coating at the mirrors. A thin film of aluminum on glass reflects 89% of the incident light (approximate value, depending on conditions) (Silver is 98%, but darkens in air due to sulfur compounds). Any laser printer contains a rotating mirror, covered with a thin layer of aluminum.

Mirrors from the optical system of the flatbed scanner. Please note that optical mirrors have metallized glass on the outside, in contrast to the usual household mirrors, where there is a reflective coating to protect behind the glass. Household mirrors give a double reflection - from the surface of the glass and from the reflective coating, which is not so critical in everyday life as the security of the reflective coating.
Electrodes of capacitor plates. Aluminum foil, separated by a layer of dielectric and tightly rolled into a cylinder, is part of the electric capacitor (however, to reduce the size of the capacitor, the foil is replaced with an aluminum coating). The fact that the aluminum oxide film is thin, durable and does not conduct current is used in electrolytic capacitors, which have enormous electrical capacitances for their dimensions.
disadvantages
Aluminum is an active metal , but in air it is covered with an oxide film, which protects the metal from destruction and hides its active nature. If aluminum is not allowed to form a stable protective film, for example, with a drop of mercury, aluminum actively reacts with water. Aluminum dissolves in an alkaline environment, try pouring aluminum foil with a pipe cleaning agent - the reaction will be violent, with release of explosive hydrogen. The chemical activity of aluminum, paired with a large difference in electronegativity with copper, makes it impossible to directly connect wires of these two metals. In the presence of moisture (there is almost always air in the air), galvanic corrosion begins to occur with the destruction of aluminum.

Two identical transformers from microwave ovens. The left one failed due to aluminum windings - the wire was burned out from the contact - aluminum is poorly soldered with soft solders, an attempt to ensure contact as well as that of the copper wire led to breakage.
Aluminum creeping. If the aluminum wire is very tight, it is deformed.
and retain a new shape - this is called "plastic deformation." If not squeeze it
so much so that it does not deform, but leave it under load for a long time - aluminum
will begin to "crawl" changing shape gradually. This dirty property leads to what is good
tightened terminal with aluminum wire after 5-10-20 years will gradually weaken and will
hang out without providing the former electrical contact. This is one of the reasons why the PUE
Prohibits thin aluminum wire for wiring electricity by consumers in
buildings. In industry, it is not difficult to ensure the regulation - the so-called "broach"
panel, when an electrician periodically checks the tightness of all terminals in the panel. At home, however, usually while the socket with the smoke does not burn - no one will attend to the quality of the contact. A bad contact is the cause of the fires.
Aluminum, in comparison with copper, is less ductile , the risk of a knife on a conductor, when removing insulation from a wire will quickly lead to a broken conductor than copper, therefore insulation from aluminum wires should be brushed off like a pencil, at an angle, and not at the end.
Interesting facts about aluminum
- Aluminum is a good reducing agent that is used to reduce other metals, such as titanium, from the state of dioxide. Theodore Gray (I strongly recommend Theodore Gray’s books “Elements. The Guide to the Periodic Table”, “Scientific Experiments with the Periodic Table”, “Experiments. Experiments with the Periodic Table.” They are very well made visually and they are not very safe, most modern benefits, and can bang.) at home conducted such an experience. In a mixture with iron oxide, aluminum powder forms a termite - an hellish mixture that burns when heated to 2400 ° C, while iron is restored and fun flows down, which is used for welding rails, otherwise this piece of iron does not heat up quickly and efficiently. Termite pencils allow you to weld wires in field conditions, and a brave commando with a termite torch will blow the handle of the strongest lock.
- Baking powder is used to make sponge cake tender and airy. The same powder is in order to make porous concrete - Aluminum + alkali.
- Aluminum is an active metal, but it is quickly covered with an oxide film that protects it from destruction. Ruby, sapphire, corundum - these are all the names of the same substance - aluminum oxide Al 2 O 3 White grinding wheels and bars consist of electrocorundum - aluminum oxide.
You can verify the activity of aluminum simple experience. Cut the aluminum foil into a glass, add the bluestone and salt, pour cold water. After some time, the mixture boils, aluminum will oxidize, reducing copper, with the release of heat. - Aluminum is a good extrusion. Instrument cases of cut and processed extruded profiles are much cheaper than cast ones.

Aluminum housing external battery for the phone. Extruded anodized painted profile. - Aluminum is very mediocre soldered with soft (tin-lead) solders, well soldered with zinc solders. When designing devices, it is worth remembering; it is easier to connect the wire to the aluminum chassis by screwing it to the pressed stand than to solder it. In solid aluminum grades (6061, 6082, 7075) you can thread the screw directly.
- Aluminum can be welded with argon welding, but high-quality weld is obtained only with TIG welding with alternating current. Continuous change of polarity crushes the film of oxides, which otherwise could get into the seam. Consider this when choosing a welding machine for the workshop if you may need to brew aluminum.
Once again, an important note. Aluminum and copper conductors cannot be directly connected! To connect conductors of copper and aluminum, use an intermediate metal, for example, a steel terminal.
Sources
In large building stores (OBI, Leroy Merlin, Castorama), aluminum profiles of various sizes and shapes are usually available. A good source can be extruded aluminum dishes - it is very cheap and there are different forms. But pay attention to the brand. If you need 6061 and even more so 7075, you will have to buy it from a company specializing in metals.
Links to parts of the manual:
1 : Conductors: Silver, Copper, Aluminum.
2 : Conductors: Iron, Gold, Nickel, Tungsten, Mercury.
3 : Conductors: Carbon, nichrome, thermostable alloys, solders, transparent conductors.
4 : Inorganic dielectrics: Porcelain, glass, mica, ceramics, asbestos, gas and water.
5 : Organic semi-synthetic dielectrics: Paper, click, paraffin, oil and wood.
6 : Synthetic dielectrics based on phenol-formaldehyde resins: carbolite (bakelite), getinax, textolite.
7 : Dielectrics: Glass fiber (FR-4), varnished cloth, rubber and ebonite.
8 : Plastics: polyethylene, polypropylene and polystyrene.
9 : Plastics: polytetrafluoroethylene, polyvinyl chloride, polyethylene terephthalate and silicones.
10 : Plastics: polyamides, polyimides, polymethyl methacrylate and polycarbonate. History of the use of plastics.
11 : Insulating tapes and tubes.
12 : Final
All Articles