When the patient is a source of income: a problem with medicines for rare diseases
How does Alexion earn so much money if her main medicine helps less than 11,000 people?
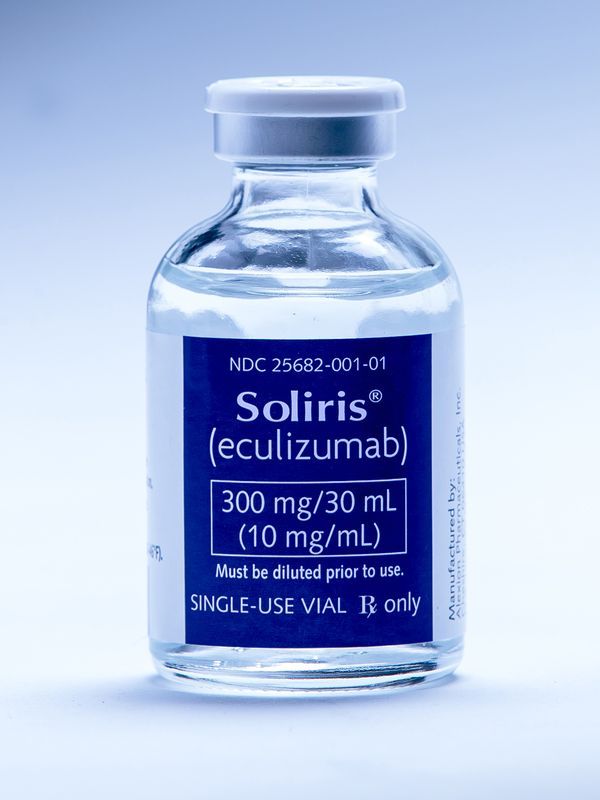
Kerry Owens, a doctor from Oklahoma City who specializes in kidney problems, was taken aback by a call on her mobile phone in September 2015. She and a team of specialists treated a woman who had recently become a mother from the nearby town of Enid , whose health began to deteriorate after giving birth. Doctors conducted countless tests, but could not determine the cause of the problems. For a time, they were worried about the fact that it could be an extremely rare and deadly blood disease, an atypical hemolytic-uremic syndrome (aHUS), from which 1 in 500,000 people suffer every year. They prescribed the medicine Soliris [with the active ingredient eculizumab], which was recently approved for use. But her health continued to deteriorate, and they stopped using the medication.
Owens called the sales representative of the company Alexion Pharmaceuticals Inc., producing "Soliris" in New Haven. Soliris is one of the most expensive drugs in the world, its annual rate is from $ 500,000 to $ 700,000 [in the USA - approx. trans.].
The seller argued with the methods of treatment. He insisted that Owens continue to use Soliris, quickly speaking to the phone information about the state of the internal organs of the mother, which the doctor did not give to the manufacturer of the medicine. How do you know that? Thought Owens. She tracked the patient's condition along with several hematologists and the presentation of the seller did not impress her. “I was completely taken aback by how persistent and arrogant he was,” says Owens. “Never, before or after this incident, have I experienced anything like it.”
Alexion is a vigorously developing company in the market of "orphan drugs" [drugs for the treatment of rare diseases, the production of which is unprofitable; US law provides for the provision of certain benefits to pharmaceutical companies producing these drugs - approx. trans.]. This is a fast-growing niche in the pharmaceutical industry. In the US, medication is given the status of "orphan" when it is needed by no more than 200,000 people across the country. Orphan drugs took a disproportionately large share of the drug market in 2014, 41%, according to a study by Johns Hopkins University. By 2022, worldwide sales of orphan drugs should double to reach $ 209 billion, according to forecasts by consulting company Evaluate Ltd.
These drugs have helped millions of people. Patients with aHUS, for example, had to undergo kidney dialysis for years and sometimes die from fatal blood clots before Soliris appeared on the market. “This is the best medicine among those who change the result of the disease of all that I have seen since I finished my studies in 1985,” says Janluigi Ardissino, a Milan doctor who treated Solyris for more than 70 patients.

But the emergence of orphan drugs led to a geological shift in treatment costs. The average patient in the US last year, using an orphan medicine, spent $ 136,000 on it, and this cost has increased by 38% since 2010. The volume of Soliris less than a teaspoon, which is administered to the patient at a time during a 35-minute session, costs more than $ 18,000, and the patient may need 26 such sessions per year for the rest of his life. [It is interesting that in Europe you can buy a German equivalent for € 5,600 or a Swiss equivalent for € 2,400 per ampoule - approx. transl.]
The company Alexion, almost all of whose income is based on sales of this single medicine, earned unrealistic amounts. In 2016, its revenue amounted to $ 3 billion, and its market value of $ 24 billion puts it on par with such companies as HP Inc. [manufacturer of computers and printers] and Yum! Brands Inc. [specializes in fast food; trademarks: Taco Bell, KFC, Pizza Hut, WingStreet]
The need to rely on a small number of customers, each of which is potentially worth millions of dollars, leads to side effects. For years, Alexion’s sales technique was so assertive that aggressive calls to doctors can be considered the most innocent of misconduct. The boundaries of ethics intersected on a routine basis, which worried many people working there - this can be seen from interviews with 20 current and former employees, as well as 2000 pages of internal documentation.
Last November, Alexion announced an internal investigation into sales practices. As a result, it became known from the press release that the company’s management could not “find the right tone” for sales. Within a few months, the company was left by the chairman of the board of directors and co-founder Leonard Bell, its executive director, financial director and head of the control service. In March, the company hired Ludwig Hentson, an industry veteran, as executive director, who shuffled control and appointed the head of culture “to redefine the organization’s culture with an emphasis on integrity, trust, and following the rules,” was written in a letter to Bloomberg. On May 23, the company announced the departure of the commercial director, the head of research, the head of the HR department and the new financial director.
Hentson refused an interview for this article and did not answer specific questions, so it is not entirely clear what caused such a strong reorganization. But the company's past seems to catch up with her, especially abroad, where the company earns up to 60% of its income - and where, according to former employees, it behaved in a blatant manner. On May 8, Brazilian police broke into the company’s offices in São Paulo as part of an investigation into its commercial activities.
Public uproar over the price of life-saving drugs for years has been shaking the entire planet. But much less attention is paid to the insane events of the Glengarry Glen Ross level [an American play and its screen version about the difficulties of working desperate realtors - approx. trans.], occurring in companies like Alexion, whose medicine costs more than most new homes, and in some parts of the world is delivered under armed guard.
Until the early 1980s, pharmaceutical companies for the most part ignored the niche of 7000 orphan diseases. There was no commercial sense to deal with fatal, but rare diseases such as Huntington's disease or muscular dystrophy , when widespread and chronic diseases such as arthritis, heart disease and diabetes provided a good flow of patients and insurance companies willing to pay bills.
To draw attention to the ignored areas, Congress in 1983 passed the Orphan Drugs Act, promising drug grants to federal drug manufacturers, tax deductions and seven years of exclusive sales of new drugs for rare diseases (for ordinary drugs, this period is limited to three years). In the following 34 years, about 600 orphan drugs were approved in the US, compared with 10 drugs in the ten years preceding the adoption of the law.
But the government-protected monopolies, together with desperate patients, led to the emergence of today's drug prices. Genzyme Corp. began this trend in 1991, demanding $ 150,000 per year for the treatment of Gaucher disease , which weakens bones and internal organs. In 2016, Biogen Inc began to take $ 750,000 for the first year of using Spinrase medicine to treat spinal muscular atrophy. “Many drug manufacturers perceive the situation as an empty signed bank check, and demand as much for the medicine as they consider possible,” says Rina Conti, associate professor of health and economics at the University of Chicago.

Leonard Bell, former director of Alexion, in 2016
Despite the controversial pricing situation, it is politically considered unprofitable to flirt with the Orphans Drugs Act. The law very effectively allows to find and focus the best scientific minds on the treatment of long-ignored diseases. It is believed that such drugs should cost more than drugs for common diseases, because companies need to repel research and development and make money on a small customer base. Government health programs, moaning from the high cost of drugs, have to figure out how to bargain with companies or make drugs available only to the most needy patients.
In many ways, Bell is an excellent example of how drug subsidies should work. When he founded Alexion in 1992, he did not have the chance to take a lot of risk. Then he was 33 years old, he worked as a cardiologist, he had three children aged from 1 to 7 years and a position at Yale University under a contract with a limited term. Bell was very interested in such an immune response, as a complementary cascade that helps blood get rid of damaged cells and bacteria. Sometimes this defensive reaction can harm a person, for example, when rejecting a transplanted organ. If he could figure out how to limit the cascade in certain cases, Bell believed, he could solve many medical problems.
It was difficult to find funding for the study, and Bell barely remained afloat. “As with most things in biotechnology, six months after leaving Yale, everything began to fall apart,” he told Bloomberg in 2015.
Alexion began working with another company in an attempt to change the internal organs of pigs so that they could be transplanted to people. Although the attempt was unsuccessful, she helped Alexion obtain funding from private investors and the government to continue research into the relationship of the immune system and blood cells.
Bell brought the company to the stock exchange in 1996. Years of research were needed before the new products were introduced to the market, but investors were willing to take risks in the light of possible profits if Alexion could reveal the secrets of the complement system - Barry Luc, former vice president of finance and administrative affairs, recalls this.
In 2002, Alexion had a breakthrough. A British researcher showed that one of the therapies really helped patients suffering from a rare blood disorder, paroxysmal nocturnal hemoglobinuria (PNH), in which the patient's complement system attacks the red blood cells. About 35% of patients die within 5 years after diagnosis. When Soliris was approved by US regulators for the treatment of PNH in 2007, it took 15 years and $ 850 million to bring the drug to the market. In 2011, Soliris was also approved for the treatment of aHUS.
In 2007, Wall Street analysts were impatiently awaiting the announcement of the market value of Soliris. Most believed that it would cost more than $ 100,000. But Alexion counted all the factors, for example, the savings of patients on trips to the hospital and blood transfusion . When the company announced a treatment price of $ 389,000 per year during a teleconference, one analyst from Credit Suisse Group AG was so taken aback that when it was his turn to ask a question, he said: “Sorry, I have no words. Could you repeat that number? ”
Soliris saved the lives of patients such as Michelle King from Canada. When she turned 21 in 1988, she was knocked down by weakness on a New Zealand walking tour. After two years of tests and medical techniques, she was diagnosed with PNH. She began a blood transfusion to restore the red blood cells and take steroids to slow down the attacks of the immune system on her body. It helped a little, but she had very little strength left, and steroids made her irritable, at the same time leading to swelling of her facial features. She tried to work in administrative positions to pay bills. “During the day, I sometimes went home to sleep, because I felt very tired,” she says.
In 2005, she came to the test of the drug Soliris, which does not cure PNH, but is able to contain symptoms. “From the very first dose, my health changed,” says King, who has since been using the drug. The energy returned, the swelling of the face disappeared, she took up horse riding. "For me, this is a magic medicine."
The company immediately increased sales efforts. “In 2007, the main question of the company was how to build a business on this? Doesn't only a few hundred people in the world suffer from PNG? ”Said the former CEO of Alexion, David Hallall, in an interview with Bloomberg in 2015. "We have not slept for 4-5 years of nights."
For new employees, the sales culture seemed too tense. Managers forced them to challenge the opinion of doctors, many of whom had never seen patients with such rare diseases, and “turn no to yes”, as one of the sellers, who left in 2016, recalls. If the doctors did not believe that the patient was sick enough to prescribe such an expensive medicine, the sellers should have warned the doctors that their patient might die.
The company carefully monitored key details - for example, the number of tests conducted by doctors in the market. Vendors distributed entangled spreadsheets with thousands of lines containing information about potential patients, including birth dates, symptom data, doctors, and hospitals. Sometimes patients were identified by initials.
A team of nurses worked together with salespeople. Pharmaceutical companies often hire professional medical staff to help with complex treatment cases. But licensed practitioners with a license are generally obliged to put the interests of patients above the profits of their employers. To avoid conflicts, most drug manufacturers separate nurses from vendors. And in Alexion, the medical staff reported directly to the sales department, and the task of seizing and retaining patients was often assigned to them, for it was they who had access to the patients.
Several former employees of the company talked about how during Friday’s salesmen’s meetings, managers gathered salespeople and nurses together to discuss customers. If someone finished taking Soliris, managers would rush at the nurse who was working with this patient: What did you do to prevent the patient from peeling off the medicine? Did you tell the patient that if you stop treatment, he can earn a fatal thrombus? Have you tried to refer the patient to another doctor who could resume treatment? “The flames flared beneath you, and the sweat ran down your back,” says one of the nurses, who had previously worked with the company for a long time, asked to remain anonymous because of fear of retribution.
Stacy is a 43-year-old housewife from Vancouver. In 2004 she was diagnosed with PNH and she began a blood transfusion. When Soliris appeared, she really wanted to try it. But the results of blood tests showed only minor improvements. When she told the nurse from Alexion that she and the doctor decided to stop the treatment, her sister began to call her and discourage her from doing so.
“I felt that they were trying to intimidate me by saying, 'Oh my god, you must not stop, you can make a blood clot and die,'” recalls Stacy. - I say: But the medicine does not help. I know it's all about dollars. ”
Possessing medicine for only two very rare diseases, Alexion has long been confronted with the question that plagues most orphan drug companies: how to find these rare patients and send their doctors to our medicine? (One of the earliest PR techniques of these companies was advertising their rare diseases in the TV series Doctor House). To find these “needles in a haystack,” as Bell called such patients, sellers spent most of their time talking to doctors, urging them to look for symptoms and persuaded them to undergo testing for rare diseases. Alexion intended to convince doctors to carry out tests for PNH and aHUS more often - and to find ways to get into the results of tests that are usually available only to the patient, doctor and laboratory.
Salespeople had to force doctors to send tests to preferred “partner laboratories,” as evidenced by several former employees of the company and internal documents. Unbeknownst to the patients and many doctors, several of these “preferred” laboratories agreed with Alexion to provide them with copies of the analyzes. The name of the patient was removed from the copies in order to avoid violating the laws. But sometimes they contained everything else — age, gender, zip code, hospital name, doctor's name, and test results. And it gave salespeople the opportunity to find patients who otherwise would have been difficult to find.
When the PNG and AHUS results were received at Alexion, the diagnostic team, about five people, passed the information to the sales department, and then she reached the doctor on the list. “It was like landing in Normandy,” says a former client relations officer. Pharmaceutical companies have been seeking access to data from laboratories for years, but they resisted, says Adam Tanner, a full-time author at the Harvard Institute of Numerical Sociology and author of Our Bodies, Our Data [Our Bodies, Our Data]. In 2010, the behavior of laboratories changed. They tried to find ways to increase slimming profits, and, hiding behind the help of drug manufacturers in their research, began selling unnamed test results to data aggregators and directly to pharmaceutical companies.
Alexion - , . , , , Laboratory Corp. of America Holdings, , LabCorp, Quest Diagnostics Inc. Even Mayo Medical Laboratories, Mayo Clinic.
, , Alexion, , , : « , , , ». 16 , , , , - . .
- . Quest Diagnostics Mayo , , . LabCorp Alexion.
, , . . 2015 Alexion 75 , .
(NORD), 1983 -, . Alexion NORD , , . « », – , - NORD, Alexion. « ».
Alexion , . , NORD. , , Alexion, . Alexion NORD , Alexion. Alexion , , NORD . NORD , . « , », – .
, , , . , , . NORD, , , .
Alexion . , - .
. , Alexion , . , , « – », , , . , [ – . trans.].
, Alexion . , , , Alexion, 2014 , , Alexion .
2012 AFAG. AFAG , Alexion 1,672 ($500 000) 2014-2015 . 30% AFAG, , . 2016 2,675 . . , AFAG Alexion, . AFAG, Alexion. , , Alexion AFAG – .
2014 , Alexion «». . 2016 600 , $200 , . . – 0,0003% – 30% 2013 2014 .
, , Alexion AFAG , – , . , , , – , , . 2,2 . , . AFAG 8 . , AFAG . , « », « ». 16 , Alexion .
Alexion . , , , , .
, Alexion . , , , , . . « , – . – ».
, , , . – ( Alexion ) ( 2013 - ) Twitter:
« , . !»
, , - , [ ]. , , - – . .
, - Alexion . Barclays Plc : « ». 18% , 2016 , 2%.
. , – , , . , , Leerink Partners LLC, , - - 11 000 . « , , – , – ».
All Articles